Genotypic Diversity among Salmonella Typhi Isolated from Children Living in Informal Settlements in Nairobi, Kenya
Abstract
The persistence of multidrug-resistant (MDR) Salmonella Typhi (S. Typhi) is a challenge especially in regions where typhoid is endemic. Surveillance of circulating genotypes of MDR S. Typhi is crucial in typhoid acute cases and carriers. This study aimed to investigate genotypic diversity of S. Typhi from symptomatic and asymptomatic children in endemic settings in Nairobi, Kenya. Symptomatic and asymptomatic individuals’ ≤ 16 years were recruited at four health facilities and tested for typhoid through stool cultures. The S. Typhi isolates were subjected to antibiotic susceptibility testing to investigate multidrug resistance. The MDR S. Typhi isolates’ DNA was extracted and illumina sequenced. Raw reads were de novo assembled and analyzed by pathogen-watch. From the 90 sequenced isolates, 60 (67%) were confirmed to be S. Typhi (sequence Type 1 and genotype 4.3.1). Out of the 60 S. Typhi strains; 39 (65%) had plasmids, from these 38 (97%) had IncHI1 plasmids alone. Out of the 60, 59 (98%) S. Typhi isolates had blaTEM-1D. Point mutations conferring reduced susceptibility to quinolones were detected in 42 (70%) of S. Typhi isolates, from these; 14 (33%) had gyrA S83Y , and 28 (67%) gyrB S464F genes, respectively. This study reports 4.3.1 (H58) as the most dominant S. Typhi genotype responsible for spread of MDR phenotypes carried on IncHI1 plasmids. Presence of MDR S. Typhi with resistance genes such as blaTEM-1Dand reduced susceptibility to ciprofloxacin especially among asymptomatic individuals, reiterates the need for use of typhoid conjugate vaccine among vulnerable children as a control and prevention measure against typhoid.
Author Contributions
Copyright © 2024 Susan Mutile Kavai, et al
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Typhoid fever caused by S. Typhi is a significant global health challenge, especially in low- and middle-income countries (LMICs) where it is endemic. According to the World Health Organization (WHO), typhoid fever accounts for 11–20 million cases worldwide each year, resulting in approximately 140,000 deaths1. In 2017, sub-Saharan Africa (SSA) reported 1.2 million cases of typhoid fever, leading to around 29,000 deaths2. In Kenya, the incidence rate of typhoid fever among children aged ≤ 8 years is 520 per 100,000 person-years of observation3.
Previous studies have identified MDR S. Typhi strains (isolates resistant to ampicillin, chloramphenicol and co-trimoxazole) in Kenya from acute cases 4, 5 and their presence in the environment continuously undermines the effectiveness of antibiotic therapy. Another major public health concern is typhoid fevers chronic carriage status which may be attributed to MDR S. Typhi in some cases6. Recurrent excretion of S. Typhi in urine and stool by carriers also contributes to the disease's transmission in the community hence the need for vaccine therapy 7, 8, 9. Although mass vaccination has been shown to significantly reduce the disease burden of typhoid fever 10, 11, it has not been widely implemented in many LMICs, including Kenya 12, 13.
Despite the endemic nature of typhoid fever in LMICs like Kenya, these countries often lack access to high-quality diagnostic tools such as whole genome sequencing, which can accurately identify and confirm circulating MDR S. Typhi genotypes. Previous studies have demonstrated that the MDR H58 lineage of S. Typhi has been responsible for multidrug-resistant typhoid fever over the past three decades in SSA and Southeast Asia 14, 15. Over the last twenty years, the MDR H58 lineage has been documented in various countries in Asia and Africa, accounting for a significant burden of typhoid fever 14, 15, 16.
Multidrug resistance in S. Typhi is primarily associated with the acquisition of large transmissible plasmids 8, 9 some of which can range in size from approximately 100 to 110 MDa and are absent in drug-susceptible strains 14. A study conducted in Thailand reported that 80% of the isolated MDR S. Typhi strains carried a self-transmissible plasmid of over 98 MDa, with the main replicon identified as incHI114.
S. Typhi has been reported to carry antimicrobial resistance genes (AMR), such as blaTEM-1(ampicillin), sul1; dfr7 (co-trimoxazole), and catA1 (chloramphenicol) 5, 17. Resistance to quinolones in S. Typhi has been associated with point mutations in the quinolone resistance determining region, specifically in the genes housing the DNA topoisomerase IV enzymes 5, 21.
Previous studies in Africa and Asia have focused on MDR S. Typhi associated with symptomatic individuals. Although the typhoid carriage state is also quite prevalent in these typhoid endemic regions, there is limited published data on MDR S. Typhi from asymptomatic individuals 6, 7. Our study aimed at analyzing the genotypic diversity; phylogenetic comparison of our isolates and those of other countries in Africa and Asia where typhoid is endemic, AMR genes and plasmids associated with MDR S. Typhi isolated from symptomatic and asymptomatic children in informal settlements in Nairobi, Kenya.
Materials and Methods
Study site and participants
We analyzed archived S. Typhi isolated from stool samples of participants enrolled in a case-control study from November 2013 to November 2018. A detailed description of the study population, including participant recruitment, sample collection and microbiology procedures have previously been documented18. Briefly, stool samples were collected from children aged ≤ 16 years living in Kibera and Mukuru informal settlements which are 6.6km and 20km from Nairobi city centre respectively.
Inclusion and exclusion criteria
The symptomatic individuals (cases) were recruited into the study if ≤ 16 years and presenting to study health facilities with a history of fever ≥38°C for the last three days and/or with three or more diarrhoea episodes. The cases were excluded from the study if they had taken antibiotics in the last 3 days. Asymptomatic (controls) children were recruited into the study if they presented to the clinics for vaccinations and/or with non-typhoid related symptoms.
Isolate revival and antibiotic susceptibility testing
Archived S. Typhi isolates previously confirmed through serology techniques (polyvalent O and monovalent antisera (9, d and vi) (Remel ltd, Europe), and frozen in Tryptone soy broth (Oxoid, Basingstoke, UK) with 15% glycerol (Span scTM Chemie, India) in a -800C freezer, at Kenya Medical Research institute (KEMRI) microbiology labs, were revived on Salmonella-Shigella agar, then sub-cultured on Mueller Hinton agar (Both from Oxoid, Basingstoke, UK) to confirm their viability. The Kirby Bauer disc diffusion method was used for antibiotic susceptibility testing (AST). For this, a loop full of the one discrete colony was emulsified in normal saline and the suspension adjusted to 0.5 MacFarland.
A sterile swab was then used to spread the suspension onto Mueller Hinton agar (Oxoid, Basingstoke, UK) prior to dispensing the antibiotic discs. Isolates were tested for susceptibility to amoxicillin -clavulanic acid (AMC, 30 µg), ampicillin (AMP, 10 µg), azithromycin (AZM, 15 µg), cefotaxime (CTX, 30 µg), cefpodoxime (CPD, 10 µg), ceftazidime (CAZ, 30 µg), ceftriaxone (CRO, 30 µg), chloramphenicol (CHL, 30 µg), ciprofloxacin (CIP, 5 µg), gentamicin (GEN, 10 µg), kanamycin (KAN, 30 µg), nalidixic acid (NAL, 30 µg), co-trimoxazole (SXT, 25 µg), and tetracycline (TET, 30 µg), all from Oxoid Basingstoke, UK. Escherichia coli ATCC 25922 was used as the control organism for the AST. The 2022 Clinical and Laboratory Standards Institute guidelines (CLSI M 100)19 were applied when interpreting AST results. At minimum, we defined multidrug resistance as combined resistance to ampicillin, chloramphenicol, and co-trimoxazole.
Genotypic characterization
The Zymo Research Quick-DNA Fungal/Bacterial kit (California, USA) was used for genomic DNA extraction following the instructions provided. A Nanodrop reader (Thermo Fisher, MA, USA) was used to estimate DNA quality and quantity. Sequencing was conducted at SeqCoast Genomics (New Hampshire, USA) using the Illumina (Nextseq2000, USA) sequencing platform.
The quality of the raw reads was assessed using FastQC v0.11.920followed by de novo assembly using the Shovill pipeline v1.1.0 https://github.com/tseemann/shovill. The genome size and read lengths were estimated from the reads and adapters were trimmed prior to assembly using SPAdes. Minor assembly errors were corrected by mapping reads back to contigs, and contigs were removed if they were too short and or had too low coverage. Final FASTA with parseable annotations were produced and renamed accordingly for ease of reference.
Genome upload and analysis
Genome assemblies were uploaded to https://pathogen.watch/ and multi-locus sequence typing (MLST) done using the http://mlst.warwick.ac.uk/mlst/dbs/Senterica database. S. Typhi serotypes and genotypes were confirmed using the Salmonella In Silico Typing Resource (SISTR) and https://github.com/ katholt/genotyphi databases, respectively. Phylogenetic trees to determine genotypic relationships between our isolates and those previously reported in Africa and Asia were generated using Micro-React, an online web-based tool. We referenced PAARSNP AMR -Library 90370 version 0.0.17 and database sourced from https://cge.cbs.dtu.dk/services/PlasmidFinder/ to identify AMR genes and plasmids, respectively.
Statistical analysis
Descriptive statistical data analysis in form of counts (n) and proportions (%) was used to present comparisons in genotypes and lineages, plasmids and AMR genes associated with MDR S. Typhi between symptomatic and asymptomatic individuals.
Results
From a total of 215 archived S. Typhi, 90 (42%) were MDR, thus selected for sequencing. Of these, 3 isolates failed at library preparations and 27 (30%) were other organisms including Citrobacter sp. (n=1), Enterobacter sp. (8), E. coli (4), and other Salmonella sp. (14). The remaining 60 that were confirmed as S. Typhi were drawn equally from symptomatic (cases) 30 (50%) and asymptomatic children, 30 (50%) (controls).
MDR S. Typhi genotypes and lineages
All 60 S. Typhi isolates belonged to the 4.3.1 genotype, with lineage 2 sub lineage EA2 predominating 28 (47%); 13 (46%) symptomatic and 15 (54%) asymptomatic children respectively. Lineage 1 sub lineage EA1 18 (30%) was the next common lineage, 6 (33%) symptomatic and 12 (67%) a symptomatic: followed by Lineage 2 sub lineage EA3 14 (23%), 11 (79%) symptomatic and 3 (21%) a symptomatic, respectively. Phylogenetic analysis showed that the genotypes 4.3.1.1 and 4.3.1.2, reported in our study closely clustered with genotypes from India and Zambia (Figure 1).
Figure 1.Phylogenetic tree comparing S. Typhi genotypes from our study with those from other countries in Africa and Asia. We selected 208 S. Typhi isolates distributed across 11 African and Asian countries. All S. Typhi isolates belonging to genotype 4.3.1 appear to originate from the same branch.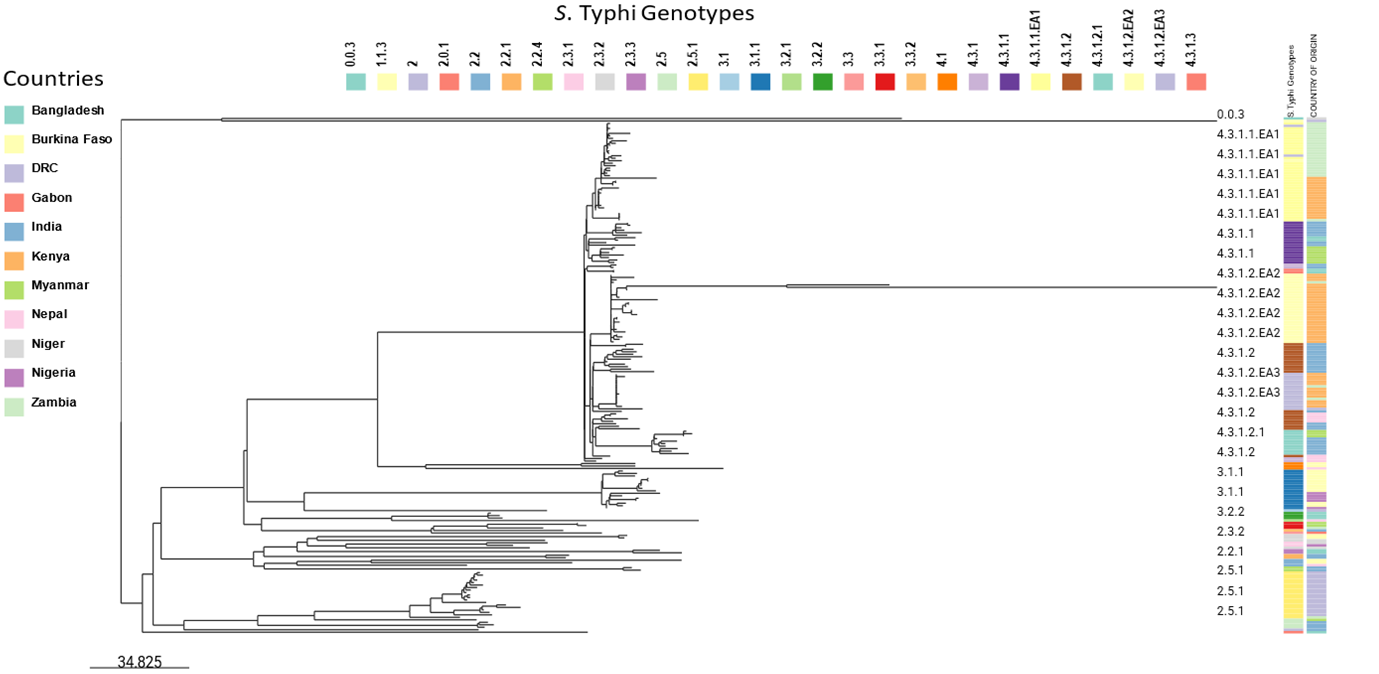
Plasmid types and their distribution
Two types of plasmids were identified in 39 (65%) of the isolates; 38 (97%) of isolates had IncHI1A/IncHI1B (R27) alone, while 1 (3%) had [IncHI1A/IncHI1B (R27) and IncFII(S), plasmids respectively. The plasmid group IncHI1A/IncHI1B (R27) alone was present in, 14 (37%) and 24 (63%) of the isolates obtained from symptomatic and asymptomatic children, respectively. The other plasmid groups, IncHI1A/IncHI1B (R27), IncFII(S) were found in a symptomatic child, 1 (3%).
AMR gene distribution
All except one S. Typhi isolate contained AMR genes that confer resistance to six antimicrobial agents. Ampicillin (blaTEM-1D), chloramphenicol (catA1), co-trimoxazole (dfr; sul1; sul2) AMR genes were each observed in 59 (98%) of the isolates, out of these 30 (51%) were from asymptomatic individuals while 29 (49%) were from symptomatic individuals, respectively. Point mutations conferring reduced susceptibility to quinolones were detected in 42 (70%) of S. Typhi isolates, 14 (33%) gyrA S83Y, and 28 (67%) gyrB S464F. Only one isolate from an asymptomatic individual showed resistance to azithromycin conferred by the mefA;msrD genes (Figure 2).
Figure 2.Antimicrobial resistance genes from S. Typhi isolated from children ≤16 years of age from Mukuru and Kibera informal settlements, Nairobi, Kenya
Discussion
Typhoid carriage has significant importance to the disease transmission in LMICs in SSA and Southeast Asia where the disease is endemic21. Countries such as Kenya suffer great disease burden both in its acute and carrier states. Shedding of MDR S. Typhi from carriers in the environment plays a major role in the persistence of typhoid fever and AMR in communities, often spreading to vulnerable individuals including children 21, 22.
Our WGS data showed that 30% of the presumed S. Typhi had been wrongly classified using serological techniques. These findings are similar to a study conducted in Kenya in 2021.This misidentification of S. Typhi highlights the need for strengthening more accurate molecular techniques in diagnostics for future studies especially in LMICs 22.
We observed that the predominant S. Typhi genotype was 4.3.1 in both symptomatic and asymptomatic individuals. The three clades observed; 4.3.1.1 EA1, 4.3.1.2 EA2 and 4.3.1.2 EA3 were all of the East Africa descent. Similar genetic clades were also found among S. Typhi isolates from Kibera in a prior study conducted in Kenya. The presence of this MDR genotype in both cases and carriers is evidence that even typhoid carriers are exposed to similar S. Typhi strains. This is an indication of human-to -human transmission of S. Typhi within this population. MDR S. Typhi carriage strains are an important source of transmission of AMR genes to the rest of the community including symptomatic children that get typhoid 23, 24.
We observed that H58 was the sole S. Typhi haplotype responsible for MDR genotypes in our isolates. A previous study on the phylo-geographical analysis of the major MDR S. Typhi H58 clade from Asia and Africa found that H58 isolates accounted for 63% of the isolates from eastern and southern Africa. African countries including; Kenya, Tanzania, Malawi, and South Africa all had H58 lineages I and II reported, confirming that H58 S. Typhi was brought to the continent more than once from South Asia25. These findings were similar to other studies conducted in Asian countries including India and Pakistan 23, 26. Similar observations were made in a separate study on phylo-geography and incidence of MDR typhoid fever in SSA26.These circulating MDR S. Typhi genotypes that originated from South East Asia could have spread overtime due to intercontinental travel, leading to multiple introductions of this genotype between the two continents26.
Our study reported S. Typhi that harboured the IncHI1 plasmids (66.7%) in both asymptomatic (63%) and symptomatic (37%) individuals. Notably the asymptomatic individuals were observed to have more S. Typhi that carried these IncHI1 plasmids which have been associated with acquisition of MDR phenotypes 26, 27. More than half of the H58 S. Typhi strains distributed well within the asymptomatic and symptomatic participants were determined to harbour the IncHI1 plasmids. Previous studies 21, 25 closely linked these plasmids to resistance to first-line antimicrobials in S. Typhi. MDR S. Typhi isolates from several Asian countries, including Vietnam28, India29, and Pakistan30 have been found to possess similar IncHI1 plasmids carry genes for resistance to almost all widely prescribed antibiotics. In a separate study, IncHI1-PST6 plasmids were found in 74% of the H58 isolates that had the MDR element. These isolates were from South Asia, Southeast Asia, and East Africa, suggesting that the IncHI1-PST6 MDR plasmid and H58 S. Typhi were transmitted across continents25. Presence of these large self-transmissible IncHI1 plasmids in our isolates is an indication of the persistence of circulating MDR S. Typhi genotypes. This further suggests that plasmid-mediated horizontal transfer has been the primary mechanism for the transmission of antimicrobial resistance determinantsin these typhoidendemic setting in Kenya31.
Our data showed presence of AMR genes (blaTEM-1D/ catA1/ dfrA7; sul1; sul2) coding for resistance to first line drugs (ampicillin, chloramphenicol and co-trimoxazole) in 98% of the S. Typhi sequenced. These AMR genes from MDR S. Typhi were also reported in another study done in Kenya22. A separate study focusing on a global scale reported AMR genes linked to MDR H58 S. Typhi strains as blaTEM-1(ampicillin resistance), dfrA7, sul1 and sul2 (resistance to trimethoprim and sulfonamides, respectively), and catA1 (chloramphenicol resistance), and strAB (streptomycin resistance). The presence of high multi drug resistance observed in our isolates could be attributed to overtime ineffectiveness of first line drugs due to their initial overuse and misuse through over the counter purchases and empirical treatment for multiple infections in Kenya 17, 22, 32.
In addition to the resistance to first line drugs, some isolates had AMR genes such as tetA; tetB,mefA; msrDfor tetracycline and azithromycin drugs. Similar findings have been reported in isolates from SSA countries such as Malawi, Kenya and South Africa 21, 25.Point mutations (gyrB S464F and gyrA S83Y) conferring reduced susceptibility to quinolones were also observed. A separate study reported similar findings of having the most frequent QRDR mutations to be changes in codon 83 of gyrA. The increase of gyrA mutations has subsequently resulted in high rates of MDR H58 strains with reduced susceptibility to fluoroquinolones. This observation likely reflects the therapeutic use of fluoroquinolones such as ciprofloxacin to treat typhoid over time in Africa and Asian countries25.
The co-occurrence of these genes in some of the isolates in both symptomatic and asymptomatic individuals is worrisome and could be as a result of misuse of these antibiotics overtime leading to their reduced efficacy. Typhoid disease caused by such S. Typhi would be difficult to treat with drugs like ciprofloxacin which is currently used to treat typhoid infections in Kenya. The presence of these genes in asymptomatic individuals is evident that carriers constantly shed S. Typhi in the community and contribute to persistence of the disease in the community 30, 32. Our study has a limitation that asymptomatic individuals were not followed up to ascertain the period they were shedding S. Typhi and whether the AMR phenotypes changed over time.
Conclusion
It is evident that H58 is responsible for MDR Typhoid fever infections since its emergence and persistent spread in SSA, and particularly Kenya. Our study reports presence of MDR H58 in both symptomatic and asymptomatic children. Presence of MDR S. Typhi in carriage poses a major challenge in prevention and control of typhoid in endemic settings. Our study therefore recommends introduction of Typhoid Conjugate Vaccine (TCV) for control of both the pathogen and MDR S. Typhi phenotypes as effective alternative tool for management of typhoid fever, as effective antimicrobial agents are either unavailable or too expensive to be afforded by patients from these endemic settings.
Ethical considerations
The parents/guardians of all our participants (≥16 years) provided a written informed consent to participate in the study. In addition, children aged 13-16 years also gave verbal assent to be recruited into the study. The consenting process was done by trained study stuff at the study sites. The mother study which provided archived samples utilized in this study obtained ethical approval from the Kenya Medical Research Institute's Scientific and Ethics Review Unit (SSC Protocol No. 2076). Additional ethical approval to conduct this study was obtained from the University of Nairobi and Kenyatta National Hospital ethical review committee study no. P950/12/2021. The National Commission for Science and Technology and Innovation in Kenya provided this study with a research license no. NACOSTI/P/22/20305. To ensure participant confidentiality and anonymity, unique research identification numbers were used to de-identify the archived samples. The Declaration of Helsinki basic principles on human research ethics were upheld during the conducting of this study.
Supplementary data
The data analysed and used for this study are available from the corresponding author on request. The S. Typhi genome FASTA files were deposited on NCBI gene bank https://submit.ncbi.nlm.nih.gov/wgs_common/report/SUB14353648.
Acknowledgements
This research was financially supported by the National Institute of Health (NIH) National institute of Allergy and Infectious Diseases (Grant Number: R01 AI099525).
Abbreviations
References
- 2.Kim J H, Choi J, Kim C, Pak G D, Parajulee P et al. (2024) Mapping the incidence rate of typhoid fever in sub-Saharan Africa. PLoS Negl Trop Dis. 38408128.
- 3.Breiman R F, Cosmas L, Njuguna H, Audi A, Olack B et al.Population-based incidence of typhoid fever in an urban informal settlement and a rural area in Kenya: Implications for typhoid vaccine use in Africa. PLoS One 2012;7. doi:. 10-1371.
- 4.Mengo D, Kariuki S, Muigai A. (2004) Salmonellaenteric serovar Typhi in Nairobi. , Kenya from 6, 393-396.
- 5.Kangogo M. (2018) Analysis of Trends. in Resistance to Fluoroquinolones and Extended Spectrum Beta-Lactams among Salmonella Typhi Isolates Obtained from Patients at Four Outpatient Clinics in Nairobi County, Kenya. Advances in Microbiology 8, 578-588.
- 6.Gunn J S, Marshall J M, Baker S, Dongol S, Charles R C et al. (2014) Salmonella chronic carriage: epidemiology, diagnosis, and gallbladder persistence. Trends Microbiol. Nov;22(11): 648-55. doi: 10.1016/j.tim.2014.06.007. Epub 22, 25065707-4252485.
- 7.Mirza S, Kariuki S, K Z Mamun, N J Beeching, H C A.Analysis of plasmid and chromosomal. DNA of multidrug-resistant Salmonella enterica serovar Typhi from Asia.Journal of Clinical Microbiology,2000;38 1449-1452.
- 8.Bentsi-Enchill A D, Hombach J.Revised global typhoid vaccination policy. Clin Infect Dis. 2019;68(Suppl 1):S31. doi:. 10-1093.
- 10.Ouedraogo A, Diarra A, Nébié I, Barry N, Kabore J M. (2023) et al.Durable Anti-Vi IgG and IgA Antibody Responses in 15-Month-Old Children Vaccinated With Typhoid Conjugate Vaccine in Burkina Faso. J Pediatric Infect Dis Soc. 12(9), 513-518.
- 11.Olaru I D, RMS Chingono, Bottomley C, Kandiye F R. (2023) et al.The effect of a comprehensive typhoid conjugate vaccine campaign on antimicrobial prescribing in children in Harare, Zimbabwe: a mixed methods study. Lancet Glob Health. 11(9), 109-23.
- 12.Nampota-Nkomba N, Nyirenda O M, Khonde L, Mapemba V. (2022) Typhoid Vaccine Acceleration Consortium team. Safety and immunogenicity of a typhoid conjugate vaccine among children aged 9 months to 12 years in Malawi: a nested substudy of a double-blind, randomised controlled trial. Lancet Glob Health. 10(9), 10-1016.
- 13.Patel P D, Patel P, Liang Y, Meiring J E, Misiri T et al. (2021) TyVAC Malawi Team. Safety and Efficacy of a Typhoid Conjugate Vaccine in Malawian Children. , N Engl 385(12), 1104-1115.
- 14.Zoe A Dyson, Duy Pham Thanh, Bodhidatta Ladaporn, Carl Jeffries Mason. (2017) Whole Genome Sequence Analysis of Salmonella Typhi Isolated in Thailand before and after the Introduction of a National Immunization Program. , PLoS Negl Trop Dis 11(1), 0005274.
- 15.Danielle J Ingle, Nair Satheesh, Hartman Hassan, Philip M Ashton. (2019) Informal genomic surveillance of regional distribution of Salmonella Typhi genotypes and antimicrobial resistance via returning travellers. PLoS Neglected Tropical Diseases,0007620.
- 16.Qian Huimin, Cheng Siyun, Liu Guoye, Tan Zhongming, Dong Chen. (2020) Discovery of seven novel mutations of gyrB, parC and parE in Salmonella Typhi and Paratyphi strains from Jiangsu Province of China. , Sci Rep 10, 7359.
- 17.Ng’eno E, Lind M, Audi A.Dynamic Incidence of Typhoid Fever over a 10-Year Period (2010–2019) in Kibera, an Urban Informal Settlement in. , Nairobi, Kenya, The American Journal of Tropical Medicine and Hygiene 109(1), 22-31.
- 18.Mbae C, Mwangi M, Gitau N. (2020) Factors associated with occurrence of salmonellosis among children living in Mukuru slum, an urban informal settlement in Kenya. , BMC Infect Dis 20, 422.
- 21.Kariuki Samuel, Zoe A Dyson, Mbae Cecilia, Ngetich Ronald, Susan M Kavai.Multiple introductions of multidrug-resistant typhoid associated with acute infection and asymptomatic carriage, Kenya eLife 2021; 10: e67852.
- 22.Kariuki S, Revathi G, Kiiru J, D M Mengo, Mwituria J.Typhoid in Kenya is associated with a dominant multidrug-resistant Salmonella enterica serovar Typhi haplotype that is also widespread in Southeast Asia. , Journal of Clinical Microbiology 2010, 2171-2176.
- 23.M D Phan, Kidgell C, Nair S, K E Holt, K A. (2009) Variation in Salmonella enterica serovar Typhi IncHI1 plasmids during the global spread of resistant typhoid fever. , Antimicrobial Agents Chemotherapy 53, 716-727.
- 24.Ochieng C, Chen J C, Osita M P, Katz L S, Griswold T. (2022) Molecular characterization of circulating Salmonella Typhi strains in an urban informal settlement in Kenya. PLoS Negl Trop Dis. 16(8), 36007074-9451065.
- 25.Wong V K, Baker S, Pickard D J, Parkhill J, Page A J et al. (2015) Phylogeographical analysis of the dominant multidrug-resistant H58 clade of Salmonella Typhi identifies inter- and intracontinental transmission events. Nat Genet. Jun;47(6): 632-9. doi: 10.1038/ng.3281. Epub 11, 25961941-4921243.
- 26.Baker S, Holt K, Vosse E van de, Roumagnac P, Whitehead S et al. (2008) High-throughput genotyping of Salmonella enterica serovar Typhi allowing geographical assignment of haplotypes and pathotypes within an urban District of Jakarta. , Indonesia. J. Clin. Microbiol 46, 1741-1746.
- 27.S E Park, D T Pham, Boinett C. (2018) The phylogeography and incidence of multi-drug resistant typhoid fever in sub-Saharan Africa. , Nat Commun 9, 5094.
- 28.Connerton P, Wain J, Hien T, Ali T, Parry C. (2000) Epidemic typhoid in Vietnam: molecular typing of multiple-antibiotic-resistant Salmonella enterica serotype Typhi from four outbreaks. , J. Clin. Microbiol 38, 895-897.
- 29.Joshi S, S K Amarnath. (2007) Fluoroquinolone resistance in Salmonella typhi and S. paratyphi A in Bangalore. , India. Trans. R. Soc. Trop. Med. Hyg 101, 308-310.
- 30.M D Phan, Kidgell C, Nair S, K E Holt, A K Turner. (2009) during the global spread of resistant typhoid fever. Variation in Salmonella enterica serovar Typhi IncHI1 plasmids , Antimicrob. Agents Chemother 53, 716-727.
Cited by (1)
- 1.Kavai Susan M., Mutai Winnie C., Mbae Cecilia, Kering Kelvin, Ng’etich Ronald, et al, 2025, Genomic insights into the role of Salmonella Typhi carriers in antimicrobial resistance and typhoid transmission in Urban Kenya, PLOS One, 20(5), e0321879, 10.1371/journal.pone.0321879
