Evaluation of Antioxidative Potential of the Biofield Energy Treated Proprietary Test Formulation on L-NAME and High Fat Diet-Induced Cardiovascular Disorders in Sprague Dawley Rats
Abstract
The aim of this experiment was to assess the antioxidative potential of the Biofield Energy Treated/Blessed Proprietary Test Formulation and Biofield Energy Treatment/Blessing per se to the animals on NG-nitro-L-arginine methyl ester hydrochloride (L-NAME) and high fat diet (HFD)-induced cardiovascular disorders in Sprague Dawley rats using various functional biomarkers. A test formulation was formulated including minerals (magnesium, zinc, copper, calcium, selenium, and iron), vitamins (ascorbic acid, pyridoxine HCl, vitamin B9, vitamin B12, and vitamin D3), cannabidiol (CBD) isolate, Panax ginseng extract, and β-carotene. The test formulation’s constituents were divided into two parts; one part was denoted as the untreated, while the other part and three group of animals received Biofield Energy Healing/Blessing Treatment remotely for about 3 minutes by a renowned spiritual leader, Mr. Mahendra Kumar Trivedi. The expression of superoxide dismutase (SOD) was elevated significantly by 198.46%, 208.73%, 191.73%, 211.75%, and 198.82% in the G5 (L-NAME + HFD + the Biofield Energy Treated test formulation), G6 (L-NAME + HFD + Biofield Energy Treatment per se to animals from day -15), G7 (L-NAME + HFD + the Biofield Energy Treated test formulation from day -15), G8 (L-NAME + HFD + Biofield Energy Treatment per se plus the Biofield Energy Treated test formulation from day -15), and G9 (L-NAME + HFD + Biofield Energy Treatment per se animals plus the untreated test formulation) groups, respectively than disease control group (G2). Moreover, the level of glutathione peroxidase (GPx) was significantly increased by 61.94%, 118.49%, 82.96%, 141.89%, and 262.02% in the G5, G6, G7, G8, and G9 groups, respectively as compared to the G2 group. Lipid peroxidase (LPO) was decreased by 14.21%, 30.98%, 38.66%, and 32.67% in the G6, G7, G8, and G9 groups, respectively than G2 group. Additionally, the level of myeloperoxidase (MPO) was decreased by 28.46%, 10.87%, 12.41%, and 13.35% in the G6, G7, G8, and G9 groups, respectively than G2. Further, the level of oxidized low density lipoprotein (LDL) was reduced by 65.38%, 65.11%, 71.53%, 79.26%, and 66.57% in the G5, G6, G7, G8, and G9 groups, respectively than G2. Besides, in heart tissues, the level of catalase (CAT) was significantly increased by 68.20%, 63.69%, 126.03%, 124.54%, and 112.23% in G5, G6, G7, G8, and G9 groups, respectively than G2 group. Moreover, in kidney tissues, the level of CAT was significantly increased by 22.48%, 23.43%, and 10.95% in the G6, G7, and G9 groups, respectively than G2. Overall, the data suggested a significant antioxidant activity by increasing the levels of SOD, CAT, GPx, and reducing the levels of LPO, MPO, and oxidized-LDL in various tissue fluids and that might be beneficial for cardiovascular disorders. Therefore, the study outcomes showed the significant slowdown the oxidative stress-related cardiovascular disease progression and its complications in the preventive treatment groups viz. G6, G7, G8, and G9.
Author Contributions
Copyright © 2021 Mahendra Kumar Trivedi, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Cardiovascular diseases (CVDs) are one of the leading cause of death worldwide 1. According to world health organisation (WHO) reported that approximately 17.9 million people died due to CVDs per year, in which specifically more than 75% death occurs in the low and middle income countries, and 80% death are due to heart attacks and strokes 2. Oxidative stress is a result of an imbalance between reactive oxygen species (ROS) and the antioxidant defense system. Superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase (CAT) are 3 major antioxidant enzymes in humans 3. The antioxidant defense systems in the body can manage the optimal levels of ROS physiologically with the help of antioxidant enzymes (AOEs) such as, cellular and mitochondrial SODs, CAT, and GPx; as well as lipid peroxidase (LPO) and myeloperoxidase (MPO), etc. 4. These antioxidant enzymes form the first line of defence against free radicals; hence, the regulation basically depends upon the oxidant status of the cell. Besides, some other factors also plays major role in their regulation, such as the enzyme-modulating action of various hormones including prolactin, growth hormone, and melatonin. Such factors may also stimulate various antioxidant enzymes by increasing their activity or by stimulating the gene expression for these enzymes 5. Therefore, in order to study the change in functional antioxidative biomarkers in serum and other tissues like heart and kidneys in presence of NG-nitro-L-arginine methyl ester hydrochloride (L-NAME) and high fat diet (HFD)-induced cardiovascular disorders in Sprague Dawley Rats, a novel test formulation was designed with the combination of vital minerals (selenium, zinc, iron, calcium, copper, and magnesium), essential vitamins (cyanocobalamin, ascorbic acid, pyridoxine HCl, vitamin B9, and cholecalciferol), and nutraceuticals (β-carotene, Ginseng, cannabidiol isolate (CBD)). All the minerals and vitamins used in the test formulation have significant functional role to provide vital physiological roles 6, 7, 8. Besides, cannabidiol itself has wide range of pharmacological profile and has been reported to role in different disorders 9, 10, while ginseng extract is regarded as the one of the best immune booster for overall immunity antioxidative activity 11. The present study was aimed to evaluate the antioxidative potential of the Biofield Energy Treated Proprietary Test Formulation and Biofield Energy Treatment per se to the animals on L-NAME and high fat diet (HFD)-induced cardiovascular disorders in sprague dawley rats using various functional biomarkers in serum and tissue (heart and kidney) homogenate.
Biofield Energy Healing Treatment has been reported with significant effects against various disorders, and defined as one of the best Complementary and Alternative Medicine (CAM) treatment approach 12, 13, 14. National Center for Complementary/Alternative Medicine (NCCAM) recommended CAM with several clinical benefits as compared with the conventional treatment approach 15. National Centre of Complementary and Integrative Health (NCCIH) accepted Biofield Energy Healing as a CAM health care approach in addition to other therapies such as deep breathing, natural products, Tai Chi, yoga, therapeutic touch, Johrei, Reiki, pranic healing, chiropractic/osteopathic manipulation, guided imagery, meditation, massage, homeopathy, hypnotherapy, special diets, relaxation techniques, movement therapy, mindfulness, Ayurvedic medicine, traditional Chinese herbs and medicines in biological systems 16, 17. The Trivedi Effect®-Consciousness Energy Healing was scientifically reported on various disciplines such as in the materials science 18, 19, agriculture science 20, antiaging 21, 22, Gut health 23, nutraceuticals 24, pharmaceuticals 25, cardiac health 26, overall human health and wellness. In this study, the authors sought to study the impact of the Biofield Energy Treatment (the Trivedi Effect®) on the given novel test formulation and Biofield Energy Treatment per se to the animals on serum antioxidants in presence of L-NAME and High Fat Diet-Induced Cardiovascular Disorders in Sprague Dawley Rats using standard ELISA assay.
Material and Methods
Chemicals and Reagents
Pyridoxine hydrochloride (vitamin B6), atorvastatin, zinc chloride, magnesium (II) gluconate, and β-carotene (retinol, provit A) were purchased from TCI, Japan. Copper chloride, cyanocobalamin (vitamin B12), calcium chloride, vitamin E (Alpha-Tocopherol), cholecalciferol (vitamin D3), iron (II) sulfate, captopril, L-NAME, and sodium carboxymethyl cellulose (Na-CMC) were procured from Sigma-Aldrich, USA. Ascorbic acid (vitamin C) and sodium selenate were obtained from Alfa Aesar, India. Cannabidiol isolate and Panax ginseng extract were obtained from Panacea Phytoextracts, India and Standard Hemp Company, USA, respectively. Standard normal chow diet and high fat diet were purchased from Altromin, USA and Research Diets, USA. For the estimation of serum antioxidative biomarker panel, specific ELISA kits were used for detection of antioxidants in serum (SOD, GPx, LPO and MPO, Oxidized LDL,) and catalase in heart and kidney tissues, were procured from CUSABIO, USA.
Maintenance of Animal
Randomly breed maleSprague Dawley (SD) rats with body weight ranges from 200 to 300 gm were used in this study. The animals were purchased from M/s. HYLASCO Biotechnology (India) Pvt. Ltd., India. Animals were randomly divided into nine groups based on their body weights consist of 15 animals of each group (at the time of induction period) and 10 animals of each group (at the time of treatment period). They were kept individually in sterilized polypropylene cages with stainless steel top grill having provision for holding pellet feed and drinking water bottle fitted with stainless steel sipper tube. The animals were maintained as per standard protocol throughout the experiment.
Consciousness Energy Healing Strategies
Each ingredient of the novel test formulation was divided into two parts. One part of the test compound did not receive any sort of treatment/Blessing and were defined as the untreated sample. The second part of the test formulation was treated with the Trivedi Effect® - Energy of Consciousness Healing Treatment/Blessing (Biofield Energy Treatment) by a renowned Biofield Energy Healer, Mr. Mahendra Kumar Trivedi under laboratory conditions for ~3 minutes. Besides, three group of animals were also Blessed (known as the Trivedi Effect®) by Mr. Mahendra Kumar Trivedi under similar laboratory conditions for ~3 minutes. The Biofield Energy Healer was located in the USA, however the test formulation were located in the research laboratory of Dabur Research Foundation, New Delhi, India. The energy transmission was done remotely, for about 3 minutes via online web-conferencing platform. After that, the Biofield Energy Treated/Blessed sample was kept in the similar sealed condition and used as per the study plan. In the same manner, the control test formulation group was subjected to “sham” healer for ~3 minutes treatment, under the same laboratory conditions. The “sham” healer did not have any knowledge about the Biofield Energy Treatment/Blessing. The Biofield Energy Treated/Blessed animals were also taken back to experimental room for further proceedings.
Experimental Procedure
Seven days after acclimatization, animals were randomized and grouped based on the body weight. The test formulation was prepared freshly prior to dosing and administered to the animals using an oral intubation needle attached to an appropriately graduated dispo sable syringe. The dose volume was 10 mL/kg in morning and evening based on body weight. The experimental groups were divided as G1 as normal control (vehicle, 0.5% w/v CMC-Na); G2 as disease control (L-NAME + HFD + 0.5% CMC); G3 as reference item (L-NAME + HFD + Captopril + Atorvastatin); G4 includes L-NAME + HFD along with untreated test formulation; G5 as L-NAME + HFD along with the Biofield Energy Treated test formulation; G6 group includes L-NAME + HFD along with Biofield Energy Treatment per se to animals from day -15; G7 as L-NAME + HFD along with the Biofield Energy Treated test formulation from day -15; G8 group include L-NAME + HFD along with Biofield Energy Treatment per se plus the Biofield Energy Treated test formulation from day -15, and G9 group denoted L-NAME + HFD along with Biofield Energy Treatment per se animals plus the untreated test formulation. The normal control animals group (G1) was receive normal drinking water and a normal diet throughout the experimental period. The animals in groups G2-G9 were received L-NAME (20 mg/kg, i.p.) and a high fat diet (HFD) throughout the experimental period. At the end of the experimental period (8 weeks treatment), the animals were sacrifice and blood was collected and separate serum subjected for antioxidants in serum (SOD, GPx, LPO and MPO, Oxidized LDL,) and catalase in heart and kidney tissues estimation.
Preparation of Sample for ELISA assay
At the end of the treatment of 8th week of the experimental period, all the animals were individually subjected for blood collection using retro-orbital route and the blood was collected in the plain vial in all the animals of different experimental groups. The serum from all the groups was stored at -20°C for further estimation. Alternatively, aliquot all the samples and store samples at -20°C or -80°C. After organ collection animals were humanely sacrificed to collect heart and kidney portion that was homogenized and subjected for the analysis of catalase using suitable ELISA method. Avoid repeated freeze-thaw cycles, which may alter the level of antioxidant level in tissues during final calculations.
Estimation of Antioxidant in Serum and Other Tissues
The serum from all the groups was subjected for the estimation of level of antioxidants such as SOD, GPx, LPO and MPO, Oxidized LDL, along with Catalase in heart and kidney tissue homogenate. All the antioxidative biomarker panel was estimation using ELISA method as per manufacturer’s recommended standard procedure. This was a quantitative method and the principle was based on the binding of antigen and antibody in sandwich manner assay.
Results and Discussion
Estimation of Serum Superoxide Dismutase (SOD)
Serum superoxide dismutase (SOD) was estimated after post treatment with the test formulation, and the data are shown in Figure 1. The SOD data suggested that the disease control (L-NAME + high fat diet (HFD) + 0.5% CMC) group (G2) showed value of SOD as 0.98 ± 0.15 U/mL, which was decreased by 62.44% as compared with the normal control (G1, 2.60 ± 0.12 U/mL). Moreover, the positive control (captopril + atorvastatin) treatment (G3) showed the level of serum SOD i.e. 2.64 ± 0.14 U/mL. The level of SOD was significantly increased by 215.98%, 198.46%, 208.73%, 191.73%, 211.75%, and 198.82% in the G4 (L-NAME + HFD + untreated test formulation), G5 (L-NAME + HFD + the Biofield Energy Treated test formulation), G6 (L-NAME + HFD + Biofield Energy Treatment per se to animals from day -15), G7 (L-NAME + HFD + the Biofield Energy Treated test formulation from day -15), G8 (L-NAME + HFD + Biofield Energy Treatment per se plus the Biofield Energy Treated test formulation from day -15), and G9 (L-NAME + HFD + Biofield Energy Treatment per se animals plus the untreated test formulation) groups, respectively, as compared to the disease control group (G2). Overall, in this experiment the Biofield Energy Treated test formulation and Biofield Energy Treatment per se significantly increased the level of antioxidant enzyme SOD in sample, which might be helpful for the management of cardiovascular disorders induced due to oxidative stress. Excessive production and inadequate removal of ROS, are responsible in the pathogenesis of various cardiovascular diseases, including atherosclerosis, hypertension, etc. 27. Another study reported that SOD is the first-line of defense antioxidant enzyme against deleterious effects of oxy-radicals in all the living cells. It breaks down the most dangerous free radical superoxide anion to molecular oxygen and hydrogen peroxide and prevents subsequent formation of hydroxyl radicals and plays an important role in the cellular antioxidant mechanism 28. Thus, Biofield Energy Treatment would be the best alternative treatment approach to treat stress induced cardiovascular dysfunctions using improved anti-oxidation action.
Figure 1.The effect of the test formulation on the level of serum superoxide dismutase (SOD) in Sprague Dawley rats. G1 as normal control (vehicle, 0.5% w/v CMC-Na); G2 as disease control (L-NAME + high fat diet (HFD) + 0.5% CMC); G3 as reference item (L-NAME + HFD + Captopril + Atorvastatin); G4 includes L-NAME + HFD along with untreated test formulation; G5 as L-NAME + HFD along with the Biofield Energy Treated test formulation; G6 group includes L-NAME + HFD along with Biofield Energy Treatment per se to animals from day -15; G7 as L-NAME + HFD along with the Biofield Energy Treated test formulation from day -15; G8 group includes L-NAME + HFD along with Biofield Energy Treatment per se plus the Biofield Energy Treated test formulation from day -15, and G9 group denoted L-NAME + HFD along with Biofield Energy Treatment per se animals plus the untreated test formulation. Values are presented as mean ± SEM (n=10).
Estimation of Serum Glutathione Peroxidase (GPx)
The effect of the Biofield Energy Treated test formulation and Biofield Energy Treatment per se on the level of serum glutathione peroxidase (GPx), is shown in Figure 2. The disease control (L-NAME + high fat diet (HFD) + 0.5% CMC) group (G2) showed the value of GPxas 4.08 ± 0.44 nmol/min/mL, which was decreased by 37.78% as compared with the normal control (G1, 6.55 ± 0.43 nmol/min/mL) group. However, positive control (captopril + atorvastatin) treatment group (G3) showed an increase the level of GPx in serum by 103.03% i.e. 8.28 ± 0.83 nmol\min/mL as compared to the G2 group. The level of GPx was significantly increased by 54.86%, 61.94%, 118.49%, 82.96%, 141.89%, and 262.02% in the G4 (L-NAME + HFD + untreated test formulation), G5 (L-NAME + HFD + the Biofield Energy Treated test formulation), G6 (L-NAME + HFD + Biofield Energy Treatment per se to animals from day -15), G7 (L-NAME + HFD + the Biofield Energy Treated test formulation from day -15), G8 (L-NAME + HFD + Biofield Energy Treatment per se plus the Biofield Energy Treated test formulation from day -15), and G9 (L-NAME + HFD + Biofield Energy Treatment per se animals plus the untreated test formulation) groups, respectively, as compared to the disease control group (G2). Further, the expression of GPx was elevated by 41.05%, 18.11%, 56.16%, and 133.71% in the G6, G7, G8, and G9 groups, respectively as compared to the untreated test formulation (G4) group. Glutathione peroxidase-1 (Gpx1) plays a crucial role in cellular defense by converting hydrogen peroxide (H2O2) and organic hydroperoxides to non-reactive products. Lack of GPx1 that accelerates a cardiac-specific hypertrophy and dysfunction in angiotensin II that leads to hypertension 29. Overall, in this study the Biofield Energy Treated test formulation and Biofield Energy Treatment per se significantly increased the antioxidant function by increasing the level of GPx, which could be beneficial in the cardiovascular patients.
Figure 2.The effect of the test formulation on the level of Serum glutathione peroxidase (GPx) in Sprague Dawley rats
Estimation of Serum Lipoperoxidase (LPO)
The level of serum lipid peroxidase (LPO) was measured in all the experimental groups and the data are shown in Figure 3. The disease control (L-NAME + high fat diet, HFD + 0.5% CMC) group (G2) group showed the value of LPO as 14.57 ± 0.93 µM, which was decreased by 23.60% as compared with the normal control (G1, 11.79 ± 0.33 µM) group. While, the positive control (captopril + atorvastatin) treatment group (G3) decreased the level of LPO by 17.43% i.e. 12.03 ± 0.64 pg/mL as compared to the G2 group. The level of LPO was decreased by 17.98%, 14.21%, 30.98%, 38.66%, and 32.67% in the G4 (L-NAME + HFD + untreated test formulation), G6 (L-NAME + HFD + Biofield Energy Treatment per se to animals from day -15), G7 (L-NAME + HFD + the Biofield Energy Treated test formulation from day -15), G8 (L-NAME + HFD + Biofield Energy Treatment per se plus the Biofield Energy Treated test formulation from day -15), and G9 (L-NAME + HFD + Biofield Energy Treatment per se animals plus the untreated test formulation) groups, respectively, as compared to the disease control group (G2). Moreover, the level of LPO was reduced by 15.85%, 25.22%, and 17.91% in the G7, G8, and G9 groups, respectively as compared to the untreated test formulation (G4) group. Lipid peroxidation is the process in which the membrane bound enzymes, proteins, and receptors are inactivated through loss of cell membrane integrity 30. Lipid per-oxidation can affects lipoproteins, cellular membranes, and other molecules containing the lipids in accordance with the oxidative stress. The process of LPO was initiated due to several reasons like acute and chronic stress, cellular damage, altered metabolism, etc. It leads to various diseases like atherosclerosis, kidney damage, Parkinson's disease, preeclampsia etc. 31. Overall, here the Biofield Energy Treated test formulation and Biofield Energy Treatment per se significantly reduced the level of lipid peroxidation end product, malondialdehyde (MDA) level, which could be beneficial in the cardiovascular symptoms.
Figure 3.The effect of the test formulation on the level of serum Lipoperoxidase (LPO) in Sprague Dawley rats.
Estimation of Serum Myloperoxidase (MPO)
The effect of the test formulation and Biofield Energy Treatment per se was estimated by measuring the level of serum myloperoxidase (MPO), and the results are shown in the Figure 4. The disease control (L-NAME + high fat diet, HFD + 0.5% CMC) group (G2) showed value of MPO as 0.43 ± 0.03 ng/mL, which was increased by 78.02% as compared with the normal control (G1, 0.24 ± 0.02 ng/mL) group. Further, the positive control (captopril + atorvastatin) treatment (G3) showed decreased serum MPO level by 25.68% i.e., 0.32 ± 0.03 ng/mL as compared to the G2 group. The level of MPO was decreased by 15.03%, 28.46%, 10.87%, 12.41%, and 13.35% in the G4 (L-NAME + HFD + untreated test formulation), G5 (L-NAME + HFD + the Biofield Energy Treated test formulation), G6 (L-NAME + HFD + Biofield Energy Treatment per se to animals from day -15), G7 (L-NAME + HFD + the Biofield Energy Treated test formulation from day -15), G8 (L-NAME + HFD + Biofield Energy Treatment per se plus the Biofield Energy Treated test formulation from day -15), and G9 (L-NAME + HFD + Biofield Energy Treatment per se animals plus the untreated test formulation) groups, respectively, as compared to the disease control group (G2). Similarly, MPO level was decreased by 16.86% in the G5 group as compared to the untreated test formulation (G4) group. The MPO is an indicator for leukocyte infiltration, which is commonly found in inflamed tissue such as chronic processes like neurodegenerative diseases and atherosclerosis 32. Therefore, in this experiment the Biofield Energy Treated test formulation and Biofield Energy Treatment per se significantly reduced the level of MPO, which could be beneficial in the cardiovascular disease conditions.
Figure 4.The effect of the test formulation on the level of serum Myloperoxidase (MPO) in Sprague Dawley rats.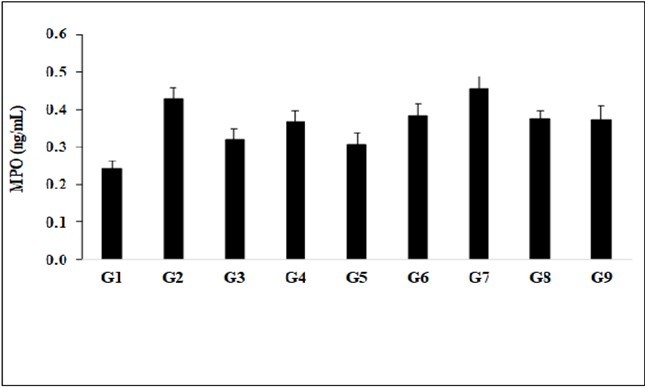
Estimation of Serum Oxidized Low Density Lipoprotein (LDL)
The effect of the test formulation and Biofield Energy Treatment per se was estimated using the level of Oxidized Low Density Lipoprotein (LDL); the results are shown in Figure 5. The level of oxidized LDL in the disease control (L-NAME + high fat diet, HFD + 0.5% CMC) group (G2) was 28.70 ± 3.12 pg/mL, which was increased by 414.71% as compared with the normal control (G1, 5.58 ± 1.25 pg/mL). Further, the positive control (captopril + atorvastatin) treatment (G3) showed decreased serum oxidized LDL level by 75.38%, 7.07 ± 1.03 pg/mL as compared with the G2. The level of oxidized LDL was decreased by 42.15%, 65.38%, 65.11%, 71.53%, 79.26%, and 66.57% in the G4 (L-NAME + HFD + untreated test formulation), G5 (L-NAME + HFD + the Biofield Energy Treated test formulation), G6 (L-NAME + HFD + Biofield Energy Treatment per se to animals from day -15), G7 (L-NAME + HFD + the Biofield Energy Treated test formulation from day -15), G8 (L-NAME + HFD + Biofield Energy Treatment per se plus the Biofield Energy Treated test formulation from day -15), and G9 (L-NAME + HFD + Biofield Energy Treatment per se animals plus the untreated test formulation) groups, respectively, as compared to the disease control group (G2). Besides, the level of oxidized LDL was decreased by 40.15%, 39.67%, 50.78%, 64.13%, and 42.21% in the G5, G6, G7, G8, and G9 groups, respectively as compared to the untreated test formulation (G4) group. Various studies have demonstrated an atherogenic role of oxidized-LDL in the progression of atherosclerotic cardiovascular disease (ASCVD) 33, 34, 35. Overall, here the Biofield Energy Treated test formulation and Biofield Energy Treatment per se has significantly reduced the level of oxidized LDL in serum sample, which could be suppressed the free-radical levels and simultaneously reduce the risks of cardiovascular diseases by antioxidative potentials.
Figure 5.The effect of the test formulation on the level of serum oxidized low density lipoprotein (LDL) level in Sprague Dawley rats.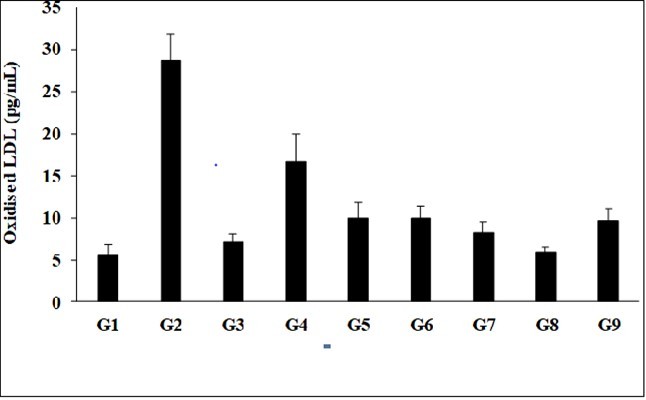
Estimation of Catalase (CAT) in Heart
Catalase enzyme activity in heart tissue was estimated in the presence of Biofield Treated test formulation and Biofield Energy treatment per se to the animals, and graphically shown in the Figure 6. The level of CAT enzyme in the disease control (L-NAME + high fat diet, HFD + 0.5% CMC) group (G2) was 194.53 ± 13.62 nmol/min/mL, which was decreased by 18.70% as compared with the normal control (G1, 239.26 ± 27.42 nmol/min/mL). Further, the positive control (captopril + atorvastatin) treatment (G3) increased the level of CAT by 82.81% as 355.62 ± 31.84 nmol/min/mL as compared with the G2. The level of CAT enzyme was increased by 86.18%, 68.20%, 63.69%, 126.03%, 124.54%, and 112.23% in the G4 (L-NAME + HFD + untreated test formulation), G5 (L-NAME + HFD + the Biofield Energy Treated test formulation), G6 (L-NAME + HFD + Biofield Energy Treatment per se to animals from day -15), G7 (L-NAME + HFD + the Biofield Energy Treated test formulation from day -15), G8 (L-NAME + HFD + Biofield Energy Treatment per se plus the Biofield Energy Treated test formulation from day -15), and G9 (L-NAME + HFD + Biofield Energy Treatment per se animals plus the untreated test formulation) groups, respectively, as compared to the disease control group (G2). Besides, the level of CAT was increased by 21.40%, 20.61%, and 14% in the G7 (L-NAME + HFD + the Biofield Energy Treated test formulation from day -15), G8 (L-NAME + HFD + Biofield Energy Treatment per se plus the Biofield Energy Treated test formulation from day -15), and G9 (L-NAME + HFD + Biofield Energy Treatment per se animals plus the untreated test formulation) groups, respectively, as compared to the untreated test formulation (G4) group. One research work in mice explored that the overexpression of cardiac-specific CAT, that detoxifies the excess H2O2, and thus protect from oxidative stress and delayed cardiac aging 36. Another study demonstrated that the antioxidant enzymes as potential targets in cardioprotection and treatment of various cardiovascular diseases 37. Overall, in this experiment the Biofield Energy Treated test formulation and Biofield Energy Treatment per se significantly reduced the level of oxidative free-radical in heart tissues, which could be suppressed the oxidative stress conditions and simultaneously reduce the risks of cardiovascular diseases.
Figure 6.The effect of the test formulation on the level of catalase activity in heart tissues in Sprague Dawley rats.
Estimation of Catalase (CAT) in Kidney
Catalase (CAT), is a powerful antioxidant enzyme that scavenge free-radical to the body. Besides, it has a powerful antioxidant support, anti-aging, and anti-degenerative effects, enhanced overall life-span, fat reduction, and prevention of DNA damage due to various stress factors 38. CAT assay in kidney homogenate was estimated in the presence of Biofield Treated test formulation and Biofield Energy treatment per se to the animals, which was measured in all the experimental groups and was graphically presented in the Figure 7. The level of CAT enzyme in the disease control (L-NAME + high fat diet, HFD + 0.5% CMC) group (G2) was 352.69 ± 17.11 nmol/min/mL, which was increased by 20.06% as compared with the normal control (G1, 441.18 ± 24.14 nmol/min/mL). Further, the positive control (captopril + atorvastatin) treatment (G3) showed the level of CAT as 299.83 ± 14.59 nmol/min/mL as compared with the G2. The level of CAT enzyme was increased by 8.33% and 9.18% in the G6 (L-NAME + HFD + Biofield Energy Treatment per se to animals from day -15) and G7 (L-NAME + HFD + the Biofield Energy Treated test formulation from day -15) groups, respectively, as compared to the disease control group (G2). Besides, the level of CAT was increased by 22.48%, 23.43%, and 10.95% in the G6 (L-NAME + HFD + Biofield Energy Treatment per se to animals from day -15), G7 (L-NAME + HFD + the Biofield Energy Treated test formulation from day -15), and G9 (L-NAME + HFD + Biofield Energy Treatment per se animals plus the untreated test formulation) groups, respectively, as compared to the untreated test formulation (G4) group (Figure 7). Therefore, it is assumed that the Trivedi Effect®-Biofield Energy Treatment based test formulation and Biofield Energy Healing Treatment per se showed good antioxidative property.
Figure 7.The effect of the test formulation on the level of catalase activity in kidney tissues in Sprague Dawley rats
Experiment includes four preventive maintenance groups (G6, G7, G8 and G9). The findings showed the significant slowdown of cardiovascular-related symptoms and also reduced the chances of disease susceptibility. Based on the overall data, it suggests that the Biofield Therapy was found to be most effective and benefited to protect from the manifestation of the existing aliments that will ultimately improve the overall health and quality of life in human.
Conclusions
The level of SOD in serum was significantly increased by 198.46%, 208.73%, 191.73%, 211.75%, and 198.82% in G5, G6, G7, G8, and G9 groups, respectively than G2. Moreover, LPO was decreased by 30.98%, 38.66%, and 32.67% in the G7, G8, and G9 groups, respectively than G2. MPO was decreased by 28.46% in the G6 group than G2. The level of oxidized LDL was significantly reduced by 65.38%, 65.11%, 71.53%, 79.26%, and 66.57% in G5, G6, G7, G8, and G9 groups, respectively than G2. Besides, the level of catalase enzyme was significantly increased by 68.20%, 63.69%, 126.03%, 124.54%, and 112.23% in G5, G6, G7, G8, and G9 groups, respectively in heart tissues than G2. However, in kidney tissues, the level of catalase was also significantly increased by 22.48% and 23.43% in G6 and G9 groups, respectively than G2. Altogether, the Biofield Energy Treated test formulation and Biofield Energy Healing Treatment (the Trivedi Effect®) per se showed fruitful results with respect to different antioxidative biomarkers in the preventive maintenance group, G6 as well as other preventive maintenance groups (G7, G8, and G9) in L-NAME and HFD-induced cardiovascular disorders rat model study. It also helped to slow down the cardiovascular disease progression and disease-related complications of the overall animal’s health. These data suggested that Biofield Energy Treatment per se and/or Biofield Energy Treated Test formulation in combination would be the best treatment strategies in order to prevent and protect from the occurrence of any type of diseases. Therefore, the Biofield Energy Treatment might act as a preventive maintenance therapy in order to maintain good health, or full restoration of health or improve the overall health and quality of life in human. This therapy might also downward the severity of acute/chronic diseases viz. Goiter, Graves' disease, thyroid cancer, fibromyalgia, Addison disease, multiple sclerosis, myasthenia gravis, aplastic anemia, psoriasis, rheumatoid arthritis, Crohn’s disease and alopecia areata, as well as multiple inflammatory disorders (ulcerative colitis, dermatitis, hepatitis, diverticulitis) and neurodegenerative disorders (mental disorders, Parkinson’s, stroke and transient ischemic attack).
Acknowledgements
The authors are grateful to Dabur Research Foundation, Trivedi Science, Trivedi Global, Inc., and Trivedi Master Wellness for the assistance and support during the work
References
- 1.Szekely Y, Arbel Y. (2018) A review of interleukin-1 in heart disease: Where do we stand today?. , Cardiol Ther 7(1), 25-44.
- 3.Yang S, Jensen M K, Rimm E B, Willett W, Wu T. (2014) Erythrocyte superoxide dismutase, glutathione peroxidase, and catalase activities and risk of coronary heart disease in generally healthy women: A prospective study. , Am J Epidemiol 180(9), 901-908.
- 4.Djordjevic J, Djordjevic A, Adzic M, Niciforovic A, Radojcic M B. (2010) Chronic stress differentially affects antioxidant enzymes and modifies the acute stress response in liver of Wistar rats. , Physiol Res 59(5), 729-36.
- 5.Duda W, Curzytek K, Kubera M, Iciek M, Kowalczyk-Pachel D et al. (2016) The effect of chronic mild stress and imipramine on the markers of oxidative stress and antioxidant system in rat liver. , Neurotox Res 30(2), 173-84.
- 6.Byrne J H, Voogt M, Turner K M, Eyles D W, McGrath J J et al. (2013) The impact of adult vitamin D deficiency on behaviour and brain function in male Sprague-Dawley rats. , PLoS One 8(8), 71593.
- 9.Peres F F, Lima A C, JEC Hallak, Crippa J A, Silva R H.Abílio VC (2018) Cannabidiol as a Promising Strategy to Treat and Prevent Movement Disorders? Front Pharmacol 9:. 482.
- 10.Nagarkatti P, Pandey R, Rieder S A, Hegde V L, Nagarkatti M. (2009) Cannabinoids as novel anti-inflammatory drugs. , Future Med Chem 1(7), 1333-1349.
- 11.Kang S, Min H. (2012) Ginseng, the 'Immunity Boost': The effects ofPanax ginsengon immune system. , J Ginseng Res 36(4), 354-368.
- 12.Maizes V, Rakel D, Niemiec C. (2009) Integrative medicine and patient-centered care. , Explore (NY) 5(5), 277-289.
- 13.Bischof M, Del Giudice E. (2013) Communication and the emergence of collective behavior in living organisms: A quantum approach. Mol Biol Int. 987549.
- 14.Cassidy C M. (2004) What does it mean to practice an energy medicine?. , J Altern Complement Med 10(1), 79-81.
- 15.Barnes P M, Bloom B, Nahin R L. (2008) Complementary and alternative medicine use among adults and children: United States. , Natl Health Stat Report 12, 1-23.
- 16.Fan K wai. (2005) National Center for Complementary and Alternative Medicine Website. , J Med Libr Assoc 93, 410-412.
- 17.Wisneski L, Anderson L. (2009) The scientific basis of integrative medicine. , Boca Raton, FL: 205.
- 18.Trivedi M K, Branton A, Trivedi D, Jana S. (2021) Effect of consciousness energy healing treatment on the metal profile and properties of tellurium. , Eng Technol Open Acc 3(5), 555623.
- 19.Mahendra K T, Alice B, Dahryn T, Snehasis J. (2021) Consciousness energy healing treatment impacted the isotopic abundance ratio of 6-Mercaptopurine (6-MP). Nov Appro Drug Des Dev. 5(5), 555673.
- 20.Trivedi M K, Branton A, Trivedi D, Nayak G, Mondal S C et al. (2015) Morphological characterization, quality, yield and DNA fingerprinting of biofield energy treated alphonso mango (Mangifera indicaL.). , Journal of Food and Nutrition Sciences 3, 245-250.
- 21.Trivedi M K, Jana S. (2021) Anti-aging activity of biofield energy treated novel proprietary test formulation by assessment of vital biomarkers in cerebrospinal fluid (CSF) in Sprague Dawley rats. , On J Neur & Br Disord 5(2).
- 22.Trivedi M K, Jana S. (2019) Evaluation of anti-aging activity of the biofield energy treated novel test formulation using SIRT1 and telomerase activity inin vitromodel. , Journal of Aging Research and Healthcare 2(4), 21-29.
- 23.Trivedi M K, Jana S. (2021) Evaluation of biofield energy healing treatment based proprietary test formulation on gut health potential in colon cancer cell line (HT-29). , J Pharmacol Clin Res 8(4), 555743.
- 24.Trivedi M K, Branton A, Trivedi D, Jana S. (2021) Isotopic abundance ratio analysis of consciousness energy healing treated folic acid. , Food Nutr Current Res 4(2), 290-295.
- 25.Trivedi M K, Branton A, Trivedi D, Jana S. (2020) The consciousness energy healing treatment and its impact on the isotopic abundance ratio analysis of flutamide. , Drug Des Int Prop Int J 3(5).
- 26.Trivedi M K, Jana.S (2019)In vitroassessment of the biofield treated test item on cardiac function using rat cardiomyocytes cell line (H9c2)viamultiparametric analysis. , Journal of Hypertension and Cardiology 2(4), 1-12.
- 27.Fukai T, Folz R J, Landmesser U, Harrison D G. (2002) Extracellular superoxide dismutase and cardiovascular disease. , Cardiovasc Res 55(2), 239-249.
- 28.Marklund S, Marklund G. (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. , Eur J Biochem 47, 469-474.
- 29.Ardanaz N, Yang X P, Cifuentes M E. (2010) Lack of glutathione peroxidase 1 accelerates cardiac-specific hypertrophy and dysfunction in angiotensin II hypertension. , Hypertension 55(1), 116-123.
- 30.Young S G, Parthasarathy S. (1994) Why are low-density lipoproteins atherogenic?. , West J Med 160(2), 153-164.
- 31.Negre-Salvayre A, Coatrieux C, Ingueneau C, Salvayre R. (2008) Advanced lipid peroxidation end products in oxidative damage to proteins. Potential role in diseases and therapeutic prospects for the inhibitors. , Br J Pharmacol153: 6-20.
- 32.Podrez E A, Abu-Soud H M, Hazen S L. (2008) Myeloperoxidase-generated oxidants and atherosclerosis. , Free Radic Biol Med 28, 1717-1725.
- 33.Gao S, Liu J. (2017) Association between circulating oxidized low-density lipoprotein and atherosclerotic cardiovascular disease. , Chronic Dis Transl Med 3(2), 89-94.
- 34.Frank M Sacks, Campos Hannia. (2003) Low-density lipoprotein size and cardiovascular disease: A reappraisal. , The Journal of Clinical Endocrinology & Metabolism 88(10), 4525-4532.
- 35.Tsimikas S. (2006) Oxidized low-density lipoprotein biomarkers in atherosclerosis. , Curr Atheroscler Rep 8, 55-61.
- 36.Yao C, Behring J B, Shao D, Sverdlov A L, Whelan S A et al. (2015) Overexpression of catalase diminishes oxidative cysteine modifications of cardiac proteins. , PLoS ONE 10(12), 0144025.
