The Impact of Nutrients on Diabetes
Abstract
Over the past 20 years, the number of persons with diabetes has more than doubled globally. The purpose of this review article is to investigate the connection between certain vitamins and diabetes. Diabetes patients have been found to have decreased amounts of certain antioxidant vitamins including A, C, and E, presumably as a result of the requirement to control oxidative stress brought on by problems with glucose metabolism. Retinol-binding protein has regulatory and adipocytokine function. Thiamine, pyridoxine, and biotin levels are also decreased in diabetics. Studies have shown that it restricts the absorption of several nutrients, such as vitamins B9 and B12, thus diabetics must frequently replenish these vitamins. Low vitamin D levels increase the risk of developing diabetes and associated complications, such as cardiovascular disease. Although some studies indicate that vitamin K supplementation can enhance glucose metabolism, it is not known if vitamin K supplementation can prevent or repair oxidative damage. Numerous studies have demonstrated the detrimental consequences of excessive vitamin supplementation. The association between a few nutrients—specifically, vitamins A, D, C, B3, B6, B9, Zn, B12, E, B1/K, and irons—and the already mentioned pathways implicated in diabetes—as well as their potential regulatory activity—will be analyzed in the review that follows.
Author Contributions
Academic Editor: Zhang Chen, University of Pennsylvania.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2024 Samer Wissam Younes
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Background
Diabetes is a group of metabolic illnesses linked to hyperglycemia that are brought on by problems with insulin production and/or action. The amount of sugar, primarily glucose, in the blood cannot be controlled by the body 1. The liver produces glucose from the food a person eats. After then, glucose is released into the blood 2. In healthy individuals, various hormones, including insulin, control blood sugar levels. The pancreas produces insulin 3. The pancreas also creates additional crucial enzymes that are immediately delivered into the gut to help with meal digestion4. Glucose is transported from the blood to cells all over the body via insulin, where it is consumed as fuel. Diabetes patients either don't generate enough insulin (type 1 diabetes), misuse insulin (type 2 diabetes), or both (many types of diabetes patients do both)5.Diabetes causes high blood sugar levels because the blood's glucose cannot properly enter the cells. This not only depletes all cells that need glucose as fuel, but it also harms several organs and tissues that are exposed to high glucose levels6. Type 1 and type 2 diabetes are the two primary forms, whereas gestational diabetes is a third variety of the disease. diabetes type 1 The pancreatic beta cells, which help the body use blood sugar (glucose) for energy, do not generate enough insulin in people with type 1 diabetes, an autoimmune condition. There is too much glucose in the blood, and cells lack energy7. Then came potentially fatal illnesses like hypoglycemia and hyperglycemia. Patients who have hypoglycemia endure disorientation, unconsciousness, and coma because their cells are not receiving enough glucose. Long-term brain glucose deficiency can potentially result in death8. diabetes type 2 A complicated endocrine and metabolic condition, type 2 diabetes. The result is a diverse, progressive illness with various degrees of insulin resistance and pancreatic beta-cell dysfunction brought on by the interplay of several hereditary and environmental factors. The majority of type 2 diabetes patients have pancreatic beta-cell dysfunction, which causes enlarged (or nonsuppressed) glucagon emission when there is hyperglycemia and probably reduced prandial GLP1 secretion9.
Methods
The following databases were searched for this review article: To find the most significant comparative research on the management of diabetes, its therapeutic options, and the effects of nutrition on patients with this disease, search engines like Google Scholar, PubMed, and Directory Open Access Journal databases. Keywords like diabetes, hyperglycemia, blood glucose, insulin, pharmacotherapy, nutrients, T2DM, T1DM, GDM, gestational diabetes, insulin resistance metabolic disorders, diabetes management, and vitamins are also used. After assessing the quality and strength of the findings, meta-analyses, systematic reviews, large epidemiological studies, and randomized control trials were used as the main sources of information where they were available. (Figure 1, Figure 2, Figure 3)
Figure 1.The chemical formula of nutrients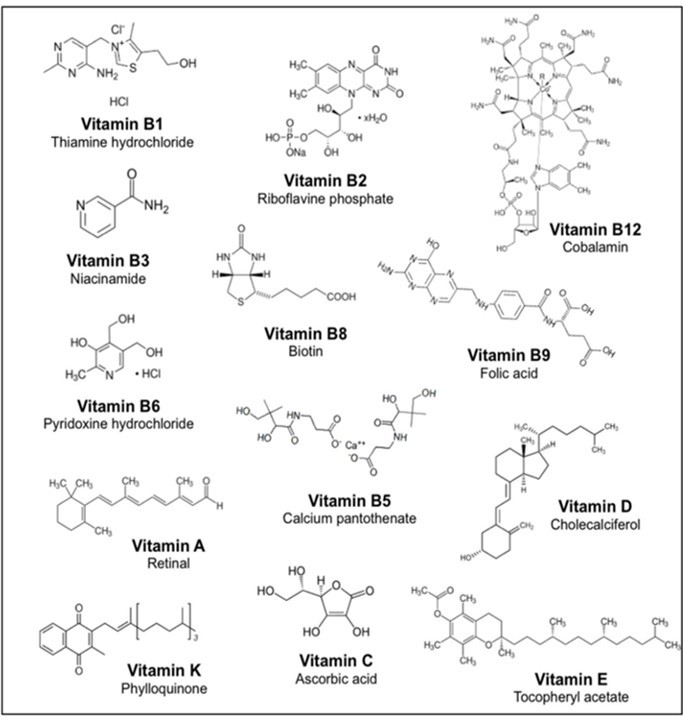
Results
Zinc
Because it encourages the manufacturing of both the hormone and its receptors, zinc plays a critical function as a mediator in the binding of growth hormone to its receptor. The amount of zinc found in pancreatic tissue, which is rich, plays a part in regulating how insulin functions. Zinc has an impact on a variety of thyroid hormone metabolic processes, including hormone synthesis, receptor activation, T4-to-T3 conversion, and the production of carrier proteins. The important relationship between zinc and leptin is demonstrated by the fact that obese patients have low zinc levels and high leptin levels. Zinc is linked to the enzyme activity that produces melatonin. Zinc absorption by the gastrointestinal tract is controlled by melatonin. Zinc has a specific effect on this procedure because 5-reductase, a zinc-dependent enzyme, is involved in the transformation of testosterone into dihydrotestosterone. Zinc is known to have significant roles in the endocrine system as a result of these relationships 10. Zinc insufficiency has been linked in certain studies to decreased insulin production and increased tissue resistance to the effects of insulin 11. Insulin is kept in the pancreatic cells as a hexamer with two zinc ions, which is released when the cells degranulate 12. In situations of Zn, Se, and Cu deficiency, which may be brought on by ingesting too many processed foods, diabetes appears to be more prevalent 13. While zinc's (Zn) significance for normal biological processes and healthy development has long been understood, Zn has also been discovered to have insulin-mimicking and anti-diabetic characteristics. These insulin-like properties have been demonstrated in isolated cells, tissues, and several animal models of type 1 and type 2 diabetes 14. Diabetes and obesity are risk factors for a lack of zinc. But up until recently, the underlying molecular processes remained unknown. The groundbreaking discovery that frequent variation in the zinc transporter SLC30A8/ZnT8 may increase susceptibility to type 2 diabetes 15 shed light on the role of zinc in diabetes. In mice with diabetes caused by streptozocin or alloxan, zinc deficiency has not been seen. It is unknown how common zinc deficiency is among diabetes patients. In one research, 9% of NIDDM patients had blood Zn levels that were low (70 /Ag/dl). In this study, the nondiabetic comparator group's blood Zn levels varied from 70 to 120 ptg/dl. The decreased serum Zn levels in diabetic individuals are likely the consequence of hyperzincuria associated with diabetes and poor intestinal Zn absorption, even if the serum Zn levels may not be connected to blood glucose levels 16, 17. A lack of zinc may also make it more likely for someone to develop diabetes, insulin resistance, glucose intolerance, and coronary artery disease. Recent studies have demonstrated that zinc has beneficial benefits on insulin resistance, glucose and lipid profiles, and those with diabetes or metabolic syndrome. For instance, a prospective study found that American women who consumed more zinc had a lower risk of developing type 2 diabetes, and additional research revealed that people with type 2 diabetes who took zinc supplements had higher levels of HDL cholesterol and lower levels of triglycerides (TG) 18. A group of NIDDM and IDDM participants had lower blood plasma Zn concentrations, according to Walter et al. 19. Diabetes patients have also been discovered to have reduced Zn levels in their lymphocytes, granulocytes, and platelets 20. Due to a rise in those with diabetes or pre-diabetic symptoms, several research have been conducted to determine ways to lessen the effects of diabetes. One of the medicines that is most usually suggested for this usage is zinc (Zn). The benefits of zinc for type 1 and type 2 diabetes have long been established. With a focus on the length of therapy and ideal zinc dosage, this study aims to provide comprehensive information regarding the effects of zinc on the different signaling pathways in multiple tissues impacted by diabetes 21. Begin-Heick et al.'s 22 discovery that Zn supplementation reduced insulin hypersecretion in ob/ob mice is yet another proof that either the diabetic rodent's dietary Zn requirement is elevated or that chronic alterations in micronutrient metabolism are linked to aberrant function. In uncontrolled trials including senior participants, a positive impact of Zn supplementation on healing venous leg ulcers has been hypothesized 23, 24. Atypical Zn, Cu, and Fe metabolism can cause chronic disorders including diabetes and its complications. Compared to Cu and Zn shortage, these pathogenic illnesses appear to be more prevalent in Cu and Fe excess. When Fe and Cu are overloaded, diabetes and its effects may be controlled using chelating drugs 25.
Vitamin E
A nutritional supplement and various foods, including nuts, seeds, vegetable oils, fruits, low-fat and fat-free milk, yogurt, cheese, and soy products, contain the fat-soluble vitamin E 28. There are eight different forms of vitamin E, but only alpha-tocopherol is found in human bodies32. In addition to its action as an antioxidant in the human body, vitamin E has been discovered to have a significant role in regulating blood sugar levels in diabetic patients. This is because, according to certain research, vitamin E therapy reduces insulin resistance and the glycemic index in people with type 2 diabetes 29, 30. In several research, vitamin E supplementation led to a noticeably lower HbA1c. Blood glucose was shown to be considerably lower following 8–10 weeks of vitamin E supplementation in addition to fasting blood glucose 26, 27. Studies have shown, among other things, that type 2 diabetes patients have lower levels of vitamin E than do other healthy persons 31. Given that several studies found lower plasma tocopherol levels in individuals with more advanced illness, its significance in diabetes is likely related to its antioxidant activity 33, 34. Vitamin E administration restored platelet thromboxane generation and aggregability in IDDM and NIDDM patients in double-blind studies 35. In rats and diabetic people, vitamin E supplementation may decrease glycosylation of glycohemoglobin, according to more recent evidence 36, 37. Both healthy control participants and 15 NIDDM patients had enhanced insulin action after receiving vitamin E supplementation (900 mg/dl for 4 months) 38. Patients with type 2 diabetes mellitus have been discovered to have lower levels of vitamin E among other vitamins 39. In the general population, high levels of -tocopherol have been linked to a lower incidence of diabetes, but not in middle-aged smokers 40, 41. A decrease in plasma tocopherol has been observed in diabetic subjects with a longer duration of the disease 42, 43, related to lipid peroxidation and cardiovascular complications 44, as well as with total cholesterol and central type obesity 45. Vitamin E's effect on the risk of diabetes and its complications is most likely due to its role as an antioxidant. However, in individuals with hemodyalisis-related diabetes, plasma tocopherol was not linked to death 46. Some vitamin supplementation trials with vitamin E and other vitamins, but not those with the haptoglobin 2-1 gene, have showed favorable effects on hypertension, blood glucose, antioxidant status, and HDL function in haptoglobin 2-2 genotype carriers 47, 48. The lipid profile and insulin sensitivity, however, were not affected, according to previous research 49, 51. In diabetic individuals who do not take vitamin E and other vitamin supplements, the risk of diabetes complications (hypertension, cardiovascular issues, central obesity, etc.) increases 51. However, as a diabetic complication, free radicals rise, increasing the diabetic patient's need for vitamin E and other antioxidants. Some research have sugested that vitamin E insufficiency is not a hallmark of diabetes complications 52. Additionally, patients with type 2 DM who get 900 mg/dl of vitamin E daily for four months will see improved insulin action 53.
Vitamin C
As medical understanding of diabetes and its care has grown, it has become clear that managing and preventing diabetic complications calls for a diversified strategy. Interest in the possible function of vitamins, minerals, and vitamin C has increased recently 54. Ascorbic acid, another name for vitamin C, is a crucial nutrient with well-known antioxidant characteristics 55. The objective of this article is to evaluate the most recent research on the effects of vitamin C on diabetes while examining both its advantages and disadvantages. Numerous research have looked at the connection between vitamin C consumption and glycemic management in diabetics 56, 57. Antioxidant levels and vitamin C concentrations have been reported to be lower in diabetes patients compared to healthy controls 58. During the first two years of the illness, it has been discovered that newly diagnosed patients of type 2 diabetes mellitus have higher levels of lipid peroxidation and lower levels of antioxidant enzymes as well as vitamins C and E 59. Plasma vitamin C levels have been shown to be negatively linked with oxidative stress, fasting and postprandial blood glucose, and glycosylated hemoglobin, but not with lipid profiles 60. It is thought that vitamin C increases insulin sensitivity, which may help with better glucose control 61. According to a research by Luo et al. (2022), higher vitamin C intake in the diet was linked to lower HbA1c levels 62. However, there are inconsistent findings, with some studies demonstrating a little or insignificant impact on glycemic management. Vitamin C's antioxidant qualities are essential for reducing oxidative stress, which is linked to the pathophysiology of issues associated with diabetes 63. Reactive oxygen species (ROS) are produced in excess as a result of chronic hyperglycemia, which contributes to tissue damage. Due to its capacity to neutralize ROS, vitamin C may help lower the risk of developing diabetic complications such nephropathy, retinopathy, and neuropathy. However, more investigation is required to pinpoint a precise causal link. Diabetes development is linked to immunological dysfunction and inflammation. The immunomodulatory properties of vitamin C may have an influence on the inflammatory processes related to diabetes 64. According to several research, people with diabetes who take vitamin C supplements may have lower levels of inflammatory markers 65. However, to validate these results and clarify the underlying processes, further in-depth clinical investigations are required. Periodontal disease and diabetes have been linked, and dental procedures and vitamin C supplements have both been demonstrated to benefit chronic periodontitis. When type 2 diabetes patients first receive a diagnosis66. Vitamin C has also been demonstrated to lower diabetes-related anxiety levels, but not stress or depressive symptoms 67. Vitamin C and E treatment for three months lowered blood pressure and glucose levels while raising levels of superoxide dismutase and glutathione 68. According to a research conducted in India, people with diabetes had lower plasma ascorbate levels and greater plasma concentrations of dehydroascorbic acid than matched control patients 69. Ascorbic acid levels in the plasma of diabetic people in England and the U.S. are apparently normal 70. In non-selected NIDDM patients, supplementing with vitamin C at a dosage of 500 mg/dl for 15 days had no effect on blood glucose levels 71. Supplementation treatment (500–1,000 mg/day) has reportedly decreased hypercholesterolemia 72 and cutaneous vascular fragility 73 in patients with poor dietary ascorbic acid consumption. Although ascorbic acid supplementation (500 mg/day) had no impact on hyperlipidemia, one trial in an unselected group revealed that it did not 74.
Iron
A macronutrient that is classified as a micronutrient that is crucial for growth and development is iron. Increased iron levels create oxidative stress, which is primarily brought on by the Fenton reaction and harms the body's regular physiological functions. The unidentified buildup of extra iron in tissue, which results in oxidative stress, is the mechanism through which inherited high iron levels induce cardiomyopathy, liver cirrhosis, and diabetes. The average adult male body has 5 grams of iron, of which 65% is found in the hemoglobin iron form at RBC. The remaining iron is utilized for cellular enzymatic activities and is stored in the liver, spleen, and bone 75. Although the exact mechanism of iron-induced diabetes is unknown, three mechanisms Insulin resistance, insulin insufficiency, and hepatic dysfunction are possible contributing factors. Oxidative stress in the pancreatic beta cells is the source of insulin insufficiency, and decreased hepatic function is the direct or indirect cause of insulin resistance 76. Ferritin levels range from 1000 to 10,000 ng/ml in people with hereditary hemochromatosis who have diabetes type 2 and circulate insulin. Diabetes in HH is brought on by both insulin resistance and insufficiency 77. Wei Boa's study revealed that type 2 diabetes was positively correlated with body stores of iron and excess dietary iron. According to the meta-analysis, supplementary iron, non-heme iron, and total iron consumption all directly affect the prevalence of type 2 diabetes. Red, the main supplementary iron source, may be the root of type 2 diabetes 78. Hepatocytes, in circumstances of food excess consumption, or macrophages, in cases of hemolytic responses, are involved in an elevated degree of insulin resistance without any injury to the tissue, which increases insulin resistance in type 2 diabetes. Additionally, a research by Forouhi et al. found that the ferritin level in plasma, which has a baseline level of 360 and is greater in those with diabetes than 758, is the greatest predictor of the development of diabetes 79.
Vitamin K
In recent years, our understanding of the physiological function of vitamin K has grown to encompass a variety of other facets of human health in addition to its control of coagulation.Phylloquinone (VK1) and menaquinone (VK2) are two naturally occurring forms of vitamin K that are available in many foods, particularly leafy greens and dairy products, and are libid-soluble vitamins 80, 81.Numerous studies have demonstrated the significance of vitamin K in controlling insulin resistance, glycemic state, and glucose metabolism. In a Spanish study 82, researchers looked at the correlation between dietary vitamin K consumption and diabetes-related indicators. Initial research revealed no correlations between the baseline values. The plasma concentrations of ghrelin, glucose-dependent insulinotropic peptide, glucagon-like peptide-1, IL-6, leptin, TNF, and visfatin were lower in people with increased vitamin K intakes after a year of follow-up. Interestingly, this study also found that higher vitamin K intakes were associated with a lower risk of acquiring diabetes mellitus 83. Another study examined the relationship between vitamin K consumption and the occurrence of type 2 diabetes mellitus over a period of around 10 years by doing a retrospective analysis of a Dutch database. The results showed a negative correlation between the intake of phylloquinone and menaquinone with the incidence of diabetes 84.
No proof was found to support the effect of phylloquinone in a comprehensive analysis by Rees et al. that looked at the effects of vitamin K intake or deficiency on cardiovascular disease, type 2 diabetes, and the metabolic syndrome. Menaquinones, according to the review, could help lower the chance of developing these illnesses 85. The use of vitamin K has been linked in several studies to insulin sensitivity, glucose metabolism, and ultimately diabetes. In the Spanish PREDIMED study, for instance, plasma concentrations of ghrelin, glucose-dependent insulinotropic peptide, glucagon-like peptide-1, IL-6, leptin, TNF, and visfatin were lower in subjects with the highest intakes after one year of follow-up compared to subjects with the lowest intakes. Baseline values did not show any associations between these markers. Increased vitamin K intakes were found to reduce the incidence of diabetes mellitus in this same research 86, 87. Following about 10 years of follow-up, a retrospective Dutch database study of vitamin K consumption and the prevalence of type 2 diabetes mellitus discovered that phylloquinone and menaquinone intakes were negatively correlated with the risk of acquiring diabetes 88. Menaquinones may play a role in lowering risk, according to Rees et al. 89, who conducted a systematic review of studies that assessed associations between vitamin K deficiency or intake and cardiovascular disease, type 2 diabetes, and the metabolic syndrome. They found no evidence of phylloquinone's effect.
Vitamin D
Although the routes are unknown, vitamin D has been shown to have a function in glucose homeostasis 90. Vitamin D3 is produced in the skin when exposed to ultraviolet B light from the sun. When skin is exposed to solar UVB radiation, 7-dehydrocholesterol in the skin is transformed to pre-vitamin D3, and this non-enzymatic, heat-dependent process culminates in the rapid conversion of pre-vitamin D3 to vitamin D3 91. The vitamin D receptor (VDR) is found in multiple organs, which implies that vitamin D metabolites may have a wide range of non-skeletal effects. Pancreatic beta-cells contain the VDR 90, 91, 92. Beta cell development and differentiation may be influenced by the active vitamin D metabolite 92. According to preclinical studies, vitamin D controls the calcium flow inside beta cells, the survival of beta cells, and insulin production. Numerous investigations have shown that rat pancreatic beta cells' capacity to release insulin in response to glucose is impacted by vitamin D deficiency, whereas vitamin D supplementation appears to enhance this capacity 91. Vitamin D regulates the immune system's early development and is crucial for the healthy emergence and maintenance of self-tolerance. Autoimmunity is more likely when vitamin D signaling is impaired, particularly in youth 93. Both animal research and observational studies have shown a correlation between a reduced level of 25-hydroxyvitamin D (25OHD) in the blood and an increased risk of type 1 diabetes 94. Low circulating vitamin D levels and type 1 diabetes are closely related 93. Type 1 diabetes is less likely to occur in children who get enough vitamin D throughout pregnancy and infancy 91, 93. Vitamin D insufficiency has been associated to metabolic syndrome and type 2 diabetes mellitus 92. The majority of cross-sectional observational studies that have looked at the connection between vitamin D status and the incidence of type 2 diabetes have concluded that there is a negative correlation between vitamin D status (25OHD concentration) and diabetes 91. In those with prediabetes, vitamin D treatment reduces the incidence of T2DM and speeds up the process by which prediabetes turns into normoglycemia 95, 96, 97. It has been demonstrated that vitamin D supplementation lowers insulin resistance 98. In accordance with ES recommendations, daily oral vitamin D dosages lower HbA1c levels over three and six months 99. Cells of the immune system and the pancreas both have vitamin D receptors. Vitamin D participates in the activity of -cell endopeptidases that depend on calcium and can act through two main pathways: either directly inducing cells to secrete insulin through an increase in intracellular calcium concentration through Ca channels or by mediating -cell calcium-dependent activation to facilitate the conversion of pro-insulin to insulin 100, 101. Vitamin D is also well known for its role in the regulation of calcium absorption. However, some interventional trials have shown that supplementing with vitamin D does not improve metabolic parameters, but rather alters adipokine concentrations, reduces pro-inflammatory cytokines like TNF-, and some other parameters like natriuretic peptide concentrations and blood pressure in diabetic patients 102, 103. Even years of follow-up were not able to reduce the incidence of diabetes, according to other research 104, 105, 106, 107, 108. The interaction of 1,25-dihydroxyvitamin D with its beta cell receptors can facilitate communication between cells. Alternately, vitamin D can improve insulin sensitivity by directly increasing insulin receptor expression and activating PPAR- (peroxisome proliferator activated receptor delta), which has been linked to the regulation of fatty acid metabolism in skeletal muscle and adipose tissue 108, 109, 110, 111. This mechanism involves the activation of 25 hydroxyvitamin D (25OHD) by 1-alpha-hydroxylase expressed in pancreatic beta cells. Additional research has revealed links between insulin resistance, -cell dysfunction, and blood vitamin D levels. Even though this interaction has only been seen in females, further research is likely required to determine whether there is a connection between sex hormones, vitamin D, adipose tissue, and -cell function 112, 113, 114, 115, 116.
Vitamin B1
A water-soluble vitamin in the vitamin B complex is thiamine, sometimes known as vitamin B1. Whole grains, enriched bread, whole cereal, legume seeds, certain fruits and vegetables, the yolk of eggs, liver, and meat all contain it. The thiamine diphosphokinase enzyme changes it into thiamin pyrophosphate, which is the physiologically active form. It is a vital component that supports the Krebs cycle, the metabolism of carbohydrates and amino acids, and is a strong antioxidant. It offers a crucial element in the development of the brain and neurotransmitters. Thus, Wernicke-Korsakoff syndrome, a neurological condition, might result from its deficit. Memory loss, ataxia, and psychosis are all signs of this illness. Beri-Beri illness and visual neuropathy can result from a deficiency 117, 118, 119. Thiamine pyrophosphate is essential for carbohydrate metabolism. It functions as a cofactor for alpha-ketoglutarate, pyruvate dehydrogenase, and transketolase. These enzymes control the Krebs cycle and glycolysis. Thiamine deficiency results in anaerobic glycolysis, which results in the creation of lactic acid or the buildup of glucose in the circulation 122. Benfotiamine, a lipid-soluble thiamine derivative, aids in the release of insulin by pancreatic cells 125. reported increased plasma thiamine levels in people with diabetes of an undefined kind and linked this to reduced thiamine tissue transport 128. Be aware that diabetic people's erythrocyte transketolase activity has been shown to be decreased, regardless of their thiamine status 129. The specific processes through which nicotinamide exerts its protective effects are unknown. A recent double-blind, randomized clinical research, however, found no evidence of nicotinamide's ability to help patients with early-onset IDDM remission 130. Numerous research have been conducted to find out how much thiamine can influence glycemic management in people with type 2 DM because of its important involvement in carbohydrate metabolism and insulin generation. In 2021, a systematic review was carried out to evaluate these research. There were six studies totaling 364 patients enrolled. They had 100–900 mg of thiamine per day. Thiamine has no effect on glycemic management in those with type 2 diabetes, according to the review. But it has a favorable impact on the lipid profile. As HDL levels rose, triglyceride levels dropped 121. Thiamine and riboflavin supplementation reduces the chance of developing gestational diabetes, according to a second trial with 3036 pregnant women 123. Nephropathy, the inforeased Diabetes macro-own bmicro-vascular complications, such as retinopathy, nephropathy, and neuropathy, due to an increase in the levels of the glycolytic metabolites glyceraldehyde 3 phosphate and fructose 6 phosphate, need further confirmation from additional studies. Reactive oxygen species build up as a result of these metabolites entering four pathways: the synthesis of advanced glycation end products (AGE), the diacylglycerol-protein kinase C (PKC), polyols, and hexosamine. By blocking three pathways (AGE, hexosamine, and the diacylglycerol (DAG)-protein kinase C (PKC) pathway), benfotiamine can prevent these issues. It is a cofactor for the enzyme transketolase, which converts pentose 5 phosphate and erythrose 4 phosphate from fructose 6 phosphate and glyceraldehyde 3 phosphate 124, 126, 127. Thiamine is required by the body in greater amounts in diabetes individuals due to its advantageous effects. Thiamine levels are lower in diabetic patients than in healthy individuals 120. Both Type 1 and Type 2 diabetes patients have been observed to have low levels of thiamine and increased renal clearance 131. Thiamine levels were lower in diabetics, with a gradual decline with albuminuria, especially so in macroalbuminuria, in a cross-sectional comparative investigation of normal controls and microalbuminuric and macroalbuminuric DM patients. A number of thiamine supplementation studies have been carried out with beneficial outcomes. Microalbuminuria was found to have a negative connection between thiamin and lipid profiles 132. For instance, it has been demonstrated that giving diabetic patients thiamine for a month lowers their glucose and leptin levels compared to controls 133. In a double-blind, placebo-controlled trial, Rabbani et al examined diabetic individuals with microalbuminuria and the subsequent decrease in urine albumin excretion following a three-month intervention 134.
Vitamin B3
Niacin, an important B vitamin, plays a substantial role in decreasing low-density lipoprotein (LDL), lowering plasma TG, and raising HDL. Niacin may be useful in controlling cholesterol levels for secondary prevention as a stand-alone therapy, especially for people who cannot tolerate statins. The underlying data, however, comes from earlier research done on a group that might not fully represent patients today. Niacin reduces the production of triglycerides, very low-density lipoprotein, and LDL cholesterol largely by limiting the release of fatty acids from adipose tissue. Niacin also appears to increase HDL cholesterol levels via boosting reverse cholesterol transport and reducing hepatic apolipoprotein A-I clearance. In the pharmaceutical industry, nicotinic acid is used to treat hyperlipidemia when given in large doses 135, 136. It is possible that these lipid-modifying processes speed up the development of atherosclerosis in people with diabetes. It is noteworthy that people with diabetes have higher amounts of cell adhesion molecules (CAM), which are crucial to the many pathways of atherogenesis. When viewed in the context of atherosclerosis processes, this increased expression of CAMs in diabetes is particularly significant. It has been demonstrated that niacin reduces the risk of cardiovascular disease and death. In addition to its well-known lipid-modifying qualities, it has shown substantial advantages in this area 137. A research was conducted to see how nicotinic acid (NA) affected the drop in plasma glucose levels. Participants were given continuous infusions of NA, somatostatin (SRIF), a combination of NA and SRIF, or a saline solution containing 0.9% NaCl throughout the duration of the experiment. The results showed that both NA and NA + SRIF infusions significantly reduced plasma non-esterified fatty acid (NEFA) concentrations, successfully bringing them down to around one-fourth of their starting levels. All of these results suggest that nicotinic acid has potential for lowering plasma glucose levels in healthy persons. This impact is thought to be mostly due to the contemporaneous decline in plasma NEFA levels. Niacin's interaction with diabetes creates a complex situation. Niacin, which is well known for its potential benefits in lipid management, but bears the risk of negative effects on glucose metabolism, which might make glycemic control in people with diabetes more difficult. Therefore, it is essential to use a patient-specific strategy and carefully weigh the risks and benefits of niacin treatment in such situations. The Coronary Drug Project found that supplementing with niacin increased the risk of type 2 diabetes mellitus in subjects with impaired fasting glucose (IFG), normoglycemia, or both. On the other hand, adverse effects have also been linked to niacin supplementation 139. According to Zhou et al, nicotinamide excess caused by an increase in the population's consumption of niacin, thiamin, and riboflavin through food fortification may be linked to oxidative stress and insulin resistance, having a detrimental impact on the emergence of complications related to type 2 diabetes mellitus 140.
Vitamin B6
The physiologically active form of vitamin B6 is pyridoxal 50-phosphate (PLP), which serves as a coenzyme in around 160 distinct enzymatic processes, the majority of which are involved in amino acid, carbohydrate, and lipid metabolism. It also has significant effects on the production and/or breakdown of a variety of neurotransmitters. By stifling oxygen reactive species (ROS) and halting the production of advanced glycation end products (AGEs), genotoxic compounds associated with aging and diabetes 141–143, PLP also serves as an antioxidant molecule. Its absence has been related to a number of clinically relevant diseases, including as autism, schizophrenia, Alzheimer's, Parkinson's, epilepsy, Down's syndrome, diabetes, and cancer, though the underlying processes are largely unclear 142. PLP deficiency has been associated to a variety of human diseases, including diabetes 141, 142, 144. Epidemiological and experimental studies established a clear negative relationship between vitamin B6 levels and diabetes as well as a preventative impact of vitamin B6 on diabetic complications 142. Rats with decreased insulin production due to PLP deficiency benefit from PLP supplementation in terms of improved gestational diabetes and avoided diabetic complications. Numerous studies showed that people with diabetes who took vitamin B6 supplements had much less neuropathy 141, 144, 145. Tanimoto and colleagues report for the first time in this issue of Metabolism that pyridoxamine (K-163) supplementation elevates blood 3-deoxyglucosone levels and urine albumin-to-creatinine ratios in KK-Ay/Ta mice, an animal model for type 2 diabetic nephropathy that develops spontaneously. The bilayer of the cell membrane, the vessel wall, the proteins, lipids, and even the cell's nucleic acids can all be harmed by this free radical if it is not quickly neutralized by an antioxidant like vitamin B6 146. Hyperglycemia and hyperketonemia, both of which produce reactive oxygen species. Vitamin B6 has an antagonistic relationship with diabetes 141. Amino acids can be converted into a source of energy by either transaminase or a deamination process, both of which are mostly performed by pyridoxal-dependent enzymes. Only PL was discovered in the plasma of diabetic rats compared to controls due to an increase in alkaline phosphatase, while vitamin B6 was largely present in the forms of PLP and PL. It is possible that more vitamin B6 was absorbed into the liver in diabetic rats since their plasma vitamin B6 levels were much lower than those of the controls. The amount of vitamin B6 would increase in the liver of diabetic rats due to the activation of AST and other transaminases. The amount of vitamin B6 would increase in the liver of diabetic rats due to the activation of AST and other transaminases 143. Whether low PLP levels are a result of, a cause of, or a combination of diabetes is not understood, though. investigations have revealed that low PLP levels can result from diabetes, whereas other investigations have showed that PLP levels are decreased by diabetes 141, 142. The plasma pyridoxal 50-phosphate (PLP) concentration is routinely used to measure vitamin B6 levels. An insufficient status of vitamin B6 is often indicated by a concentration below the threshold of 30 nmol/L 142. The consequences of pyridoxal 50-phosphate shortage on diabetes may vary. For instance, PLP could have an impact on the process that converts tryptophan into nicotinic acid since it is a cofactor of some of the enzymes involved in that system. It has been proven that the metabolites produced when this route is defective may interfere with the biological action of insulin, resulting in insulin resistance, a defining feature of type 2 diabetes. According to a hypothesis, PLP may influence insulin resistance by controlling the expression of genes related to fat tissue. According to a different notion, homocysteine levels may increase due to decreased cystathionine-b-synthase (CBS) and cystathionine-g-lyase (CGL) enzymes, both of which need PLP as a cofactor, if PLP is deficient 141, 142. It has been demonstrated that animals deficient in vitamin B6 had reduced pancreatic and blood levels of insulin. Deficiency has been connected to declining changes in the h-cells in the islets of Langerhans and impaired glucose tolerance 144. Eight months to 28 years of follow-up on 18 people with diabetes mellitus who had varied levels of steroid and vitamin B6 medication, some of whom had retinopathy, were pregnant, or had carpal tunnel syndrome. A connection between a vitamin B6 deficiency and diabetes has been demonstrated by examining the specific activity of the erythrocyte glutamic oxaloacetic transaminase and once again by the linkage with the carpal tunnel syndrome. It has been known for 10 years that CTS and a B6 deficiency are related 147. An adversarial link exists between vitamin B6 levels and diabetes, according to an analysis of the research described in the literature. According to Satyanarayana and colleagues' cross-sectional case-control research 148, the mean plasma PLP levels in T2D individuals were significantly lower than in healthy controls. Comparing the findings of Ahn and colleagues from a Korean research with those of Nix and associates from a German cohort suggested an inverse relationship between vitamin B6 levels and the development of diabetes 149, 150. The advanced clinical stage diabetes group Nix investigated showed median plasma concentrations of PLP, PN, and PL that were considerably lower in the diabetic group in comparison to the controls 150. Ahn and colleagues, who looked at diabetics with an early stage of the illness, discovered that a decrease in the mean plasma PLP level was important but not statistically significant compared to controls 149. The discovery that median plasma levels of PM, PMP, and pyridoxic acid were significantly higher in the diabetes groups than in the controls led Nix and colleagues to advance the theory that T2D may be connected to a changed activity of the enzymes involved in the interconversion of B6 vitamins 150. Another study made the assumption that reduced vitamin B6 levels in T2D individuals might be the consequence of flawed reabsorption mechanisms based on the data showing increased vitamin B6 urine clearance. Falling PLP levels have also been connected to GDM. PLP levels were lower than anticipated in 13 of 14 women with GDM who took part in a research by Bennink and Schreurs 151–153. According to results from other intervention studies, pyridoxine supplementation can reduce glycosylated hemoglobin levels in type 2 diabetics and blood glucose levels in streptozotocin-treated rats. According to studies by Kim and colleagues 154, vitamin B6 can also reduce postprandial blood glucose levels after ingesting sucrose and starch by inhibiting the activity of small-intestinal _-glucosidases. In this investigation, the role of vitamin B6 (pyridoxine) treatment in pregnant people with gestational diabetes was examined. According to the current study, vitamin B6 treatment enhanced glucose tolerance in a single, small sample. Except for the 5-minute sample, all of the individual glucose readings have significantly decreased following the B6 administration. Additionally, treatments were used to lower 38.5% of the fasting blood glucose readings to acceptable levels (90 mg per 100 ml). The blood glucose profiles that accompanied the medication as a result demonstrated a discernible improvement 155. Finally, compelling evidence demonstrates the undeniable connection between vitamin B6 and diabetes through a number of mechanisms and pathways 142. These results could indicate that diabetic animals need to eat extra vitamin B6 since diabetes might lead to a vitamin B6 deficit 143. On the other hand, this study, which was a 30-year follow-up study, found a negative correlation between the risk of diabetes with folate consumption but not with vitamin B12 or pyridoxine. It has been suggested that diabetics who don't get enough nourishment from their diets may only benefit from nutritional supplements. The benefits of these two B vitamins on the risk of acquiring diabetes would not be as great as those of folate, according to this argument, which could assist to explain why the current investigation did not find an association between vitamin B6 and B12 consumption and the incidence of diabetes 156.
Vitamin B9
Scientific evidence supports the importance of the vitamin B9 folate in preventing and controlling diabetes mellitus 157, 158. Folate participates in a number of metabolic processes, including insulin control and glucose metabolism. According to research, consuming enough folate may increase insulin sensitivity, lowering the chance of developing insulin resistance, a characteristic of type 2 diabetes 157. According to a research in Diabetes Care 2018, those with greater levels of folate had a decreased chance of getting type 2 diabetes, but folate deficiency has been linked to a higher risk of getting diabetes 161. Additionally, a systematic review and meta-analysis that was published in the American Journal of Clinical Nutrition in 2018 discovered that adults with type 1 and type 2 diabetes mellitus and prediabetes in the US had a lower risk of dying from cardiovascular disease when their dietary folate intake was higher 159, 160. This shows that dietary folate may enhance glycemic management and prevent diabetes in general. Although further investigation is required to completely understand the processes underlying folate's effects on diabetes, the available scientific evidence highlights the promise of this vitamin as a dietary component in the prevention and management of this widespread metabolic illness.
Vitamin B12
Cobalamin, the physiologically active form of vitamin B12, is a micronutrient also known as cyanocobalamin. Adenosyl cobalamin, a cofactor of the enzyme methionine synthase, and methyl cobalamin, a cofactor of mutase, are the two active forms that result from its subsequent metabolism. Yoshiatsu Takahashi et al. investigated the metabolism of vitamin B12 in 19 diabetic patients with impaired glycemic control and 15 healthy individuals. Its insufficiency may cause a variety of complications, including megaloblastic anemia, insulin resistance, hyperhomocysteinemia, defective neurotransmitter synthesis, impaired cognition, cardiovascular diseases, and neurological complications 161. They found a greater correlation between diabetes patients' blood fructosamine levels and total vitamin B12 binding ability. This demonstrates the link between glycemic management and vitamin B12 metabolism in people with diabetes mellitus 162. The effect of vitamin B12 on type 2 diabetes mellitus patients was confirmed in a randomized, multi-arm, open-labeled clinical trial involving 80 type 2 diabetes mellitus patients who were divided into 4 groups with 20 patients each and observed for 8 weeks 163. These groups who received vitamin B12 supplements demonstrated improved glycemic control and reduced insulin resistance. When analyzing the plasma concentration level of vitamin B12 in 913 pregnant women during the 26th gestational week, Jun S. Lai et al. reported a higher prevalence of gestational diabetes mellitus among people with a combined insufficiency of vitamin B12 (48%) and high folate concentration 164. A meta-analysis research comprising 1810 pregnant women, 309 of whom had gestational diabetes mellitus, revealed similar findings. The findings support a somewhat greater link between vitamin B12 deficiency and an increased risk of developing gestational diabetes mellitus 165. According to Elizabeth Kos et al., a retrospective assessment of patient medical data revealed that metformin-treated individuals had statistically lower vitamin B12 levels than non-treated patients. This suggests that type 2 diabetes individuals taking metformin have a greater prevalence of vitamin B12 insufficiency 166. Likewise, Valdos-Ramos, Roxana, et al. reported it. Their review research found a greater frequency of vitamin B12 deficiency in type 2 diabetes mellitus patients receiving metformin treatment, since metformin use for an extended period of time decreases vitamin B12 absorption, resulting in vitamin B12 insufficiency in such individuals 167. Diabetic foot ulcers are another problem brought on by a vitamin B12 deficiency. A substantially greater correlation between vitamin B12 deficiency and diabetic foot ulcers in type 2 diabetes mellitus patients was found in Mohammed Badedi et al.'s case-control study of 323 type 2 diabetes mellitus patients, including 108 patients with diabetic foot ulcers 168. Patients with type 1 and type 2 diabetes mellitus have been found to have a greater frequency of clinical and biochemical vitamin B12 insufficiency 169. Patients with type 2 diabetes mellitus frequently have insomnia, which appears to be related to higher vitamin B12 levels. The findings of a cross-sectional research by Shuyuan Xiong et al. among 418 type 2 diabetes mellitus patients, where 24.16 percent of them reported experiencing insomnia, point to an independent contribution to insomnia in type 2 diabetes mellitus patients 170.
Discussion
The number of people with diabetes rose from 108 million in 1980 to 422 million in 2014. Prevalence has been rising more rapidly in low- and middle-income countries than in high-income countries.Diabetes is a major cause of blindness, kidney failure, heart attacks, stroke and lower limb amputation.Between 2000 and 2019, there was a 3% increase in diabetes mortality rates by age.In 2019, diabetes and kidney disease due to diabetes caused an estimated 2 million deaths. This study was done to see whether diabetes and vitamin and mineral shortages were related. Three categories may be drawn from the associations: Positive connection, No connection, and Controversial are the three options. Vitamins B9, E, D, B1, K, and zinc were included in the first category (demonstrating a positive association) because they appear to affect the diabetes.The results showed that vitamin K has a significant role in glucose metabolism.The study's goal is to employ vitamin K as a medication to control type 2 diabetes mellitus 176, 177. As a consequence, vitamin K can be effective in treating patients with type 2 diabetes mellitus. Vitamin K has a critical function in lipid metabolism and lowers blood levels of HDL and triglycerides, which has a significant influence on the treatment of diabetes, particularly type 2 178. Additionally, diabetic and pre-diabetic problems are greatly impacted by vitamin K. While the study failed to demonstrate the impact of vitamin K on fasting blood glucose 179, it did have a substantial impact on insulin and glucose levels two hours after meals. As of now, a research has demonstrated the benefits of vitamin K supplementation for diabetic patients, namely in older women and men who had improved insulin resistance after taking vitamin K supplements for 36 months 180, 181. Along with its impact on bone mineral density and fracture rates, vitamin K has also been demonstrated to raise bone mineral density and decrease bone fractures in individuals with osteoporosis and Menopause 182, 183. It is said to have the ability to imitate insulin and manage blood sugar levels. In order to improve the ligands' capacity to regulate blood sugar, it has been complexed with a variety of ligands 184. In animal models of diabetes, zinc treatment has been demonstrated to improve lipid and carbohydrate metabolism. The molecular mechanism behind the insulin-like actions of Zn compounds 185, 186, 187 includes activation of the phosphatidylinositol 3-kinase (PI3-K)/protein kinase B/Akt (PKB/Akt) and extracellular signal-regulated kinase 1/2 (ERK1/2) pathways, among others. Zinc has received a lot of interest in the study and creation of efficient anti-diabetic medications due to its role in the storage and manufacture of insulin as well as its putative insulin-mimetic properties. Zinc (II) has been complexed with a number of chemical ligands as an adjuvant 188. in order to produce anti-diabetic medications with improved and/or broader scope of pharmacological effects. Zinc supplementation significantly reduced two-hour postprandial hyperglycemia (2hpp) and other glycemic indicators 189, 190. Important molecules involved in cell signaling that promote glucose control seem to be activated by zinc. Zinc also prolong the effects of insulin, regulates insulin receptors, and supports healthy lipid profiles. The development of T2DM is influenced by oxidative stress, which can be brought on by excessive copper. Abnormal Zn and Cu metabolism may contribute to diabetes complications 191, 192Because it lowers the risk of diabetic complications, vitamin E is crucial for patients with type 2 diabetes Meletus 202, 201. Although consuming vitamin E has a considerable impact on lowering the HA1c in diabetic patients, particularly type 2 DM the research did not detect a significant impact on fasting blood sugar 203, 204. Lack of vitamin E has a significant influence on diabetes because it acts as an antioxidant, which reduces long-term issues such diabetic neuropathy and other complications including cardiovascular problems 205, 206. Studies have shown that both sexes, including pregnant women, need 15 mg of vitamin E daily from either natural sources or supplements 196. Some investigations suggested that persons with vitamin E deficiencies may experience neurological issues, but the studies were unable to establish this since the deficit had been present for a long time 197. Since the brain is the organ responsible for smell and taste perception, vitamin E deficient patients may experience neurological as well as taste and smell changes 198, 199. Studies have shown that brain tissue is the tissue most impacted by vitamin E deficiency. According to research, patients with dysfunctions of taste and smell had an improvement after taking vitamin E supplements 200. Additionally, vitamin E functions as a cofactor in both cell division and death. In addition to its essential function in the immune system and central nervous system. Understanding folate's function in critical metabolic pathways is key to recognizing its possible influence on diabetes. According to research, consuming enough folate improves insulin sensitivity, which is crucial for lowering the likelihood of insulin resistance, a feature of type 2 diabetes. However, further research is necessary to fully understand the complex processes behind folate's impact on insulin sensitivity 223. Empirical data strongly supports the hypothesis that folate plays a crucial role in reducing the risk of diabetes. Notably, a 2018 study published in the journal Diabetes Care found that those with greater amounts of folate in their bodies had a considerably lower chance of acquiring type 2 diabetes. On the other hand, a low folate intake was linked to a higher risk of developing diabetes. These results highlight the potential of folate as a dietary element that may be changed to prevent diabetes 224. In addition, folate has an influence on cardiovascular health in people with diabetes in addition to reducing the risk of developing diabetes. According to a thorough systematic review and meta-analysis published in the American Journal of Clinical Nutrition in 2018, adults in the United States with type 1 and type 2 diabetes mellitus and prediabetes had a lower risk of dying from cardiovascular disease when their dietary folate intake was higher. This suggests that, in addition to improving glycemic control, folate may also improve cardiovascular outcomes in diabetes patients 225. While current scientific evidence supports the potential benefits of folate in the prevention and management of diabetes, the complex mechanisms underlying these effects call for more research. To fully utilize folate's therapeutic potential, it is imperative to have a better understanding of how it affects glucose metabolism, cardiovascular health, and insulin sensitivity in the context of diabetes 226. In conclusion, research has shown how important folate is in reducing the risk of developing diabetes mellitus and its complications. Folate is an important dietary component in managing and preventing diabetes because of its potential to improve insulin sensitivity and participation in important metabolic pathways. The correlations between folate levels and cardiovascular outcomes that have been identified further highlight the varied effects of this vitamin in the treatment of diabetes. Low serum vitamin D is linked to an increased risk of type 1 diabetes mellitus because vitamin D regulates immune system development. As ongoing research reveals the intricate mechanisms of action of folate, it is emerging as a promising adjunctive strategy in treating this common metabolic disorder. Type 1 diabetes may be influenced by low levels of vitamin D 227, 228, 229, 230. Inadequate vitamin D supplementation during infancy has been associated to an increased risk of type 1 diabetes later in life 230. Significantly lowering the chance of acquiring Type I diabetes is vitamin D supplementation 231. Low vitamin D levels are also linked to type 2 diabetes mellitus; prediabetes and diabetic individuals respond well to vitamin D treatment. There may be a link between low vitamin D levels and T2DM 232. In persons with pre-diabetes and hypovitaminosis, high-dose vitamin D improves insulin sensitivity and reduces the chance of developing diabetes 233. When diabetic patients receive vitamin D, their HbA1c will go down. Niacin (B3), B6, C and vitamin A were placed in the third group (i.e., " controversial" association). Niacin (B3) is crucial for reducing TAG and LDL while raising HDL levels. This helps to ward against atherosclerosis. Niacin may be helpful in avoiding diabetic angiopathies since atherosclerosis plays a significant role in the development of these diseases in people with diabetes mellitus. Niacin may be used to reduce blood glucose levels, according to a study 193. Thiamin is crucial for the breakdown of glucose. There is no proof, according to studies looking at the usage of thiamine in managing glucose levels in diabetics. However, they have shown progress in lowering diabetics' lipid profiles194. It has been suggested that riboflavin and thiamine can help prevent gestational diabetes 195. Even though Vitamin C may help manage diabetes, there are some restrictions that must be recognized. It is difficult to arrive to firm conclusions because to variation in study designs, demographics, doses, and outcomes measurements. Individual reactions to vitamin C supplementation may also vary depending on things like heredity, nutrition, and general health. Recent research indicates that Vitamin C may help manage diabetes by improving glucose control, reducing oxidative stress, and controlling inflammatory reactions 171, 172, 173, 174. The current corpus of evidence is still not definitive, calling for more study through well planned clinical studies. Although using vitamin C as a supplemental method in diabetes management shows potential, medical professionals must use prudence and take into account individualized treatment plans 175. Animal studies support the link between vitamin B6 deficiency and diabetes. Vitamin B6 deficiency in animals results in decreased pancreatic and blood insulin levels, impaired glucose tolerance, and noticeable changes in the pancreatic islet cells 219. Epidemiological studies reveal an inverse relationship between vitamin B6 levels and diabetes susceptibility, underscoring the need of maintaining appropriate vitamin B6 levels for effective glucose metabolism. People who have low vitamin B6 reserves are more likely to develop diabetes, whereas those who take vitamin B6 supplements regularly have better glycemic control and fewer problems from diabetes. In terms of managing diabetes, the mounting evidence emphasizes the therapeutic potential of vitamin B6 supplementation 220. However, this study offers a challenge, arguing that folate—rather than vitamin B12 or vitamin B6—may have a detrimental effect on diabetes incidence. This discrepancy emphasizes the complex interplay between multiple nutrients in the development of diabetes, with distinct B vitamins perhaps exerting differing degrees of effect 221. This study emphasizes the complex relationship between vitamin B6, specifically PLP, and diabetes. PLP plays several functions in metabolic processes, antioxidant fortification, and enzymatic orchestration, positioning it as a key actor in preserving metabolic health and reducing consequences from diabetes. The data strongly supports the maintenance of optimum vitamin B6 levels as an essential component in lowering diabetes risk and the accompanying problems, even though the precise processes await additional investigation. Folate, or vitamin B9, has attracted a lot of attention in scientific circles for its potential role in preventing and managing diabetes mellitus. As a result, the promotion of a well-rounded diet, complemented by consideration of vitamin B6 supplementation, emerges as a valuable strategy in the prevention and management of diabetes. This water-soluble B-vitamin plays a significant role in a number of metabolic pathways, including those that are essential for insulin control and glucose metabolism. Interest in the significance of folate to diabetes has been inspired by its implications in various metabolic processes 223.It's important to note that while some research indicates a possible connection between vitamin A and diabetes management, more research is required to make specific recommendations for vitamin A supplementation in people with diabetes. A healthcare professional must be consulted before using vitamin A supplements since too much vitamin A might be harmful 234. Contrarily, vitamin B12 consumption fits under category two (i.e., has no bearing on or link to olfaction). Adenosyl cobalamin and methyl cobalamin are the two active forms of vitamin B12, generally known as cyanocobalamin. Megaloblastic anemia, hyperhemocysteinemia, cognitive problems, sleeplessness, neurological problems, and cardiovascular problems can all result from a lack of it 207, 208. Since metformin decreases vitamin B12 absorption in diabetic patients, which can result in a number of issues, its shortage has been extremely common among diabetic patients using the medication. Although vitamin B12 supplementation is not often advised for diabetic patients, there is a substantial correlation between vitamin B12 deficiency and insulin resistance and glycemic control in diabetes mellitus 209, 210, 211. In type 2 diabetes mellitus patients, a lack of vitamin B12 has also been linked, independently, to insomnia 212. Additionally, people with gestational diabetes mellitus who are vitamin B12 deficient are more likely to experience it insufficiency 213. The results of this study illuminate the complex interactions between pyridoxal 5'-phosphate (PLP), the biologically active form of vitamin B6, and diabetes by highlighting its multifaceted involvement in metabolic processes, cellular protection, and its potential implications for the onset and control of diabetes 214. PLP plays a crucial part in directing around 160 enzymatic processes including the metabolism of amino acids, carbohydrates, and lipids. These interactions, which have a direct impact on how nutrients are used and how much energy is produced, are essential for the healthy operation of our physiological systems. PLP is also involved in the production and catabolism of neurotransmitters, which further emphasizes its importance for brain health. PLP performs a wide range of functions, emphasizing the possibility that any deficit in PLP levels could have profound effects on metabolism 215. In addition to its role in metabolism, PLP also wears the antioxidant hat, skillfully combating the negative effects of reactive oxygen species (ROS) and preventing the formation of advanced glycation end products (AGEs). These antioxidant qualities are crucial for preventing cellular damage, which is a major factor in age-related illnesses like diabetes. Thus, maintaining appropriate PLP levels becomes a viable tactic for slowing the development of diabetic complications 216, 217. A collection of investigations has unequivocally shown the connection between PLP deficit and diabetes. Plasma PLP levels are frequently lower in diabetics, suggesting a possible link between lower PLP levels and insulin resistance. The complex processes behind this relationship are yet unknown, however theories range from disrupted tryptophan metabolism to dysfunctional cystathionine-synthetase (CBS) and cystathionine-lyase (CGL), two enzymes that use PLP as a cofactor. Therefore, PLP deficiency may act as a catalyst for insulin resistance, a key component of type 2 diabetes 218.
Conclusion
The complex interactions between several nutrients, including zinc, iron, and the vitamins A, B1, B3, B6, B9, B12, C, D, and E, have been shown to have a role in diabetes. Although the process of diabetes has many facets, it's important to remember that this research is a first step in comprehending this complex relationship. It's crucial to remember that diabetes is influenced by a wide range of complicated circumstances. While this analysis provides insightful information, it's important to recognize that further study is required to fully understand this complex dynamic. Numerous earlier research have shed light on the probable connection between diabetes and abnormalities in the quantities of particular nutrients in the human body. These results underline how crucial it is to have these nutrients in an ideal balance for diabetes to work properly. However, given the complexity of this relationship, further study is needed to determine the particular processes at play and the extent to which nutritional control affects diabetes.
Abbreviations
1. Glucagon-like peptide-1 : GLP-1
2. T2DM : Type 2 of diabetes
3. T1DM : Type 1 of diabetes
4. Gestational diabetes mellitus (GDM)
5. Non-insulin-dependent diabetes mellitus (NIDDM)
6. Insulin dependent diabetes mellitus (IDDM)
7. HbA1c :a blood test that is used to diagnose type 2 diabetes
8. Reactive oxygen species (ROS)
9. RBC : Red Blood Cell
10. diacylglycerol (DAG)-
11. protein kinase C (PKC)
12. ow-density lipoprotein (LDL),
13. cell adhesion molecules (CAM)
14. nicotinic acid (NA)
15. non-esterified fatty acid (NEFA)
16. impaired fasting glucose (IFG)cell adhesion molecules (CAM
17. CTS carpal tunnel syndrome cystathionine-b-synthase (CBS) and cystathionine-g-lyase (CGL) plasma pyridoxal 50-phosphate (PLP)
18. oxygen reactive species (ROS)
References
- 1.Peter J Watkins, Paul L Drury, Keith William Taylor. (1990) Diabetes and its management. , Boston: Blackwell Scientific
- 3.Anjali D Deshpande, Harris-Hayes Marcie, Schootman Mario. (2008) Epidemiology of diabetes and diabetes-related complications." Physical therapy 88.11. 1254-1264.
- 6.Mark A Atkinson, George S Eisenbarth, Aaron W Michels. (2014) Type 1 diabetes. , The Lancet 383, 69-82.
- 8.Chatterjee Sudesna, Khunti Kamlesh, Melanie J Davies. (2017) Type 2 diabetes." The lancet 389.10085. 2239-2251.
- 10.Baltaci A K, Mogulkoc R, Baltaci S B. (2019) Review: The role of zinc in the endocrine system. Pak J Pharm Sci. 32(1), 231-239.
- 11.Hendricks D G, Mahoney. (1972) AW: Glucose tolerance in zinc deficient rats. , J Nutr 102, 1079-1084.
- 12.Jayawardena R, Ranasinghe P, Galappatthy P, Malkanthi R, Constantine G et al. (2012) Effects of zinc supplementation on diabetes mellitus: a systematic review and meta-analysis. Diabetol Metab Syndr. 13-10.
- 13.Bjørklund G, Dadar M, Pivina L, Doşa M D, Semenova Y et al.The Role of Zinc and. Copper in Insulin Resistance and Diabetes Mellitus. Curr Med Chem 27(39), 6643-6657.
- 14.Vardatsikos G, Pandey N R, Srivastava A K. (2013) Insulino-mimetic and anti-diabetic effects of zinc. J Inorg Biochem. Mar;120: 8-17. doi: 10.1016/j.jinorgbio.2012.11.006. Epub 2012 Dec 3. PMID: 23266931.
- 15.Fukunaka A, Fujitani Y.Role of Zinc Homeostasis. in the Pathogenesis of Diabetes and Obesity. Int J Mol Sci. 2018 Feb 6;19(2): 476. doi: 10.3390/ijms19020476. PMID: 29415457; PMCID: PMC5855698 .
- 16.Kinlaw W B, Levine A S, Morley H E, Silvis S E, McClain. (1983) CJ: Abnormal zinc metabolism in type II diabetes mellitus. , Am J Med 75, 273-277.
- 17.Song M K, Mooradian A D. (1988) Intestinal zinc transport: influence of streptozotocininduced diabetes, insulin and arachidonic acid. , Life Sci 42, 687-694.
- 18.Kim J, Lee S. (2012) Effect of zinc supplementation on insulin resistance and metabolic risk factors in obese Korean women. Nutr Res Pract. Jun;6(3): 221-5. doi: 10.4162/nrp.2012.6.3.221. Epub 30, 22808346-3395787.
- 19.Walter RM Jr, Uriu-Hare J Y, Olin K L, Oster M H, Anawalt B D et al. (1991) zinc, manganese, and magnesium status and complications of diabetes mellitus. , Diabetes Care 14, 1050-1056.
- 20.Pai L H. (1988) Prasad AS: Cellular zinc in patients with diabetes mellitus. , Nutr Res 8, 889-897.
- 21.Asghari K, Shargh Z, Fatehfar S, Chodari L, Sameei P. (2022) The impact of zinc on the molecular signaling pathways in the diabetes disease. J Trace Elem Med Biol. Jul;72: 126985. doi: 10.1016/j.jtemb.2022.126985. Epub 11, 35429747.
- 22.Begin-Heick N, Dalpe-Scott M, Rowe J. (1985) Heick HMC: Zinc supplementation attenuates insulin secretory activity in pancreatic islets of the ob/ob mouse. , Diabetes 34, 179-184.
- 23.Haeger K, Lanner E, Magnusson P O. (1974) Oral zinc sulfate in the treatment of venous leg ulcer. In Clinical Applications of Zinc Metabolism. Pories WJ, Strain WH, Hsu JM, Woosley RL, Eds , Springfield, IL, Thomas 158-67.
- 25.Zheng Y, Li X K, Wang Y, Cai L.The role of zinc, copper and iron in the pathogenesis of diabetes and diabetic complications: therapeutic effects by chelators. , Hemoglobin 32(1), 135-45.
- 26.Ward N C, Wu J H.The efect of vitamin E on blood pressure in individuals with type 2 diabetes: a randomized, double-blind, placebocontrolled trial. https://doi.org/10.1097/ 01.hjh.0000254373.96111.43 , J Hypertens 25(1), 227-34.
- 27.Bril F, Biernacki D M.Role of vitamin E for nonalcoholic steatohepa titis in patients with type 2 diabetes: a randomized controlled trial. Diabetes Care. 42(8), 1481-8.
- 28.. Modern Nutrition in Health and Disease. 10th ed Traber MG. Vitamin E. In: Shils ME, Shike M, Ross AC, Caballero B, Cousins R, eds , Baltimore, MD: Lippincott Williams 2006-396.
- 29.Paolisso G, D’Amore A.Daily vitamin E supplements improve meta bolic control but not insulin secretion in elderly type II diabetic patients. Diabetes Care. 16(11), 1433-7.
- 30.Udupa A S, Nahar P S.Study of comparative efects of antioxidants on insulin sensitivity in type 2 diabetes mellitus. , J Clin Diagn Res 6(9), 1469-73.
- 31.E P Odum, A, V C Wakwe. (2012) Antioxidant status of type 2 diabetic patients in Port Harcourt. , Nigeria. Niger, J. Clin. Pract 15(1), 55-58.
- 32.R I Henkin, J D Hoetker. (2003) Deficient dietary intake of vitamin E in patients with taste and smell dysfunctions: is vitamin E a cofactor in taste bud and olfactory epithelium apoptosis and in stem cell maturation and development?. , Nutrition 19(11), 1013-1021.
- 33.M C Polidori, Mecocci P, Stahl W, Parente B, Cecchetti R et al. (2000) Plasma levels of lipophilic antioxidants in very old patients with type 2 diabetes. , Diabetes Metab. Res. Rev 16(1), 15-19.
- 34.M O Ebesunun, E O Obajobi. (2012) Elevated plasma homocysteine in type 2 diabetes mellitus: a risk factor for cardiovascular diseases. , Pan. Afr. Med. J 12, 48.
- 35.Wu H P, Tai T Y, Chuang L M, Lin B J, Wnag J D. (1992) Teng CM: Effect of tocopherol on platelet aggregation in non-insulindependent diabetes mellitus: ex vivo and in vitro studies. Taiwan I-Hsueh-Hui. , Tsa-Chih 91, 270-275.
- 36.Ozden I, Deniz G, Tasali E, Ulusarac A, Altug T. (1989) Buyukdevrim S: The effect of vitamin E on glycosylated hemoglobin levels in diabetic rats: a preliminary report. , Diabetes Res 12, 123-124.
- 37.Ceriello A, Giugliano D, Quatraro A, Donzella C, Dipalo G et al. (1991) PJ: Vitamin E reduction of protein glycosylation in diabetes: new prospect for prevention of diabetic complications?. , Diabetes Care 14, 68-72.
- 38.Paolisso G, D'Amore A, Giugliano D, Ceriello A, Varricchio M.D'Onofrio F: Pharmacologic doses of vitamin E improve insulin action in healthy subjects and non-insulin-dependent diabetic patients. , AmJ Clin Nutr 57, 650-656.
- 39.E P Odum, A, V C Wakwe. (2012) Antioxidant status of type 2 diabetic patients in Port Harcourt. , Nigeria. Niger, J. Clin. Pract 15(1), 55-58.
- 40.Peerapatdit T, Patchanans N, Likidlilid A, Poldee S, Sriratanasathavorn C. (2006) Plasma lipid peroxidation and antioxidiant nutrients in type 2 diabetic patients. , J. Med. Assoc. Thai 89, 147-155.
- 41.M O Ebesunun, E O Obajobi. (2012) Elevated plasma homocysteine in type 2 diabetes mellitus: a risk factor for cardiovascular diseases. , Pan. Afr. Med. J 12, 48.
- 42.V K llison, P H Rondó, Oliveira A M de, F H D'Abronzo, K F Campos. (2011) The relationship between plasma alphatocopherol concentration and vitamin E intake in patients with type 2 diabetes mellitus. , Int. J. Vitam. Nutr. Res 81(1), 12-20.
- 43.C, Diabetes German, Dialysis Study Investigators. (2013) Low plasma α-tocopherol concentrations and adverse clinical outcomes in diabetic hemodialysis patients. , Clin. J. Am. Soc. Nephrol 8(3), 452-458.
- 44.Blum S, Vardi M, J B Brown, Russell A, Milman U et al. (2010) . Vitamin E reduces cardiovascular disease in individuals with diabetes mellitus and the Pharmacogenomics 11(5), 675-684.
- 45.Farbstein D, Blum S, Pollak M, Asaf R, H L Viener et al. (2011) Vitamin E therapy results in a reduction in HDL function in individuals with diabetes and the haptoglobin 2-1 genotype. , Atherosclerosis 219(1), 240-244.
- 46.Goldenstein H, N S Levy, Y T Lipener, A P Levy. (2013) Patient selection and vitamin E treatment in diabetes mellitus. , Expert Rev. Cardiovasc. Ther 11(3), 319-326.
- 47.Vardi M, Blum S, A P Levy. (2012) Haptoglobin genotype and cardiovascular outcomes in diabetes mellitus – natural history of the disease and the effect of vitamin E treatment. Meta-analysis of the medical literature. , Eur. J. Intern. Med 23(7), 628-632.
- 48.Oliveira A M de, P H Rondó, L A, F H D'Abronzo, V K Illison. (2011) The effects of lipoic acid and α-tocopherol supplementation on the lipid profile and insulin sensitivity of patients with type 2 diabetes mellitus: a randomized, doubleblind, placebo-controlled trial. Diabetes Res. , Clin. Pract 92(2), 253-260.
- 49.Suksomboon N, Poolsup N, Sinprasert S. (2011) Effects of vitamin E supplementation on glycaemic control in type 2 diabetes: systematic review of randomized controlled trials. , J. Clin. Pharm. Ther 36(1), 53-63.
- 50.Rafighi Z, Shiva A, Arab S, Yousof Mohd, R. (2013) Association of dietary vitamin C and E intake and antioxidant enzymes in type 2 diabetes mellitus patients. , Glob. J. Health Sci 5(3), 183-187.
- 51.Farbstein D, Blum S, Pollak M, Asaf R, H L Viener et al. (2011) Vitamin E therapy results in a reduction in HDL function in individuals with diabetes and the haptoglobin 2-1 genotype. , Atherosclerosis 219(1), 240-244.
- 52.Darby W J, Ferguson M E, Furman R H, Lemley J M, Ball C T.Meneely GR: Plasma tocopherols in health and disease. , Ann NYAcad Sci 52, 328-388.
- 53.Paolisso G, D'Amore A, Giugliano D, Ceriello A, Varricchio M.D'Onofrio F: Pharmacologic doses of vitamin E improve insulin action in healthy subjects and non-insulin-dependent diabetic patients. , AmJ Clin Nutr 57, 650-656.
- 54.a Sh. (2022) Vitamin C Supplementation for Diabetes Management:. , A Comprehensive Narrative Review.” Free Radical Biology and Medicine, Pergamon 14, 0891584922010292.
- 55.Bendich. (2007) The Antioxidant Role of Vitamin C.”. Advances in Free Radical Biology & Medicine, Pergamon,27 8755966886800217.
- 56.Shaun A Mason. (2021) Effects of Vitamin C Supplementation on Glycemic Control and Cardiovascular Risk Factors in People with Type 2 Diabetes: A Grade-Assessed Systematic Review and. , Meta-Analysis of Randomized Controlled Trials.” American Diabetes Association, American Diabetes Association 13, 44-2.
- 57.a S. (2019) . Impact of Rutin and Vitamin C Combination on Oxidative Stress and Glycemic Control in Patients with Type 2 Diabetes.” Clinical Nutrition ESPEN, Elsevier 14, 2405457719304887.
- 58.E P Odum, A, V C Wakwe. (2012) Antioxidant status of type 2 diabetic patients in Port Harcourt. , Nigeria. Niger, J. Clin. Pract 15(1), 55-58.
- 59.R K Sundaram, Bhaskar A, Vijayalingam S, Viswanathan M, Mohan R et al. (1996) Antioxidant status and lipid peroxidation in type II diabetes mellitus with and without complications. , Clin. Sci. (Lond) 90(4), 255-260.
- 60.Carter P, L J Gray, Talbot D, D H Morris, Khunti K et al. (2013) Fruit and vegetable intake and the association with glucose parameters: a cross-sectional analysis of the Let's Prevent Diabetes Study. , Eur. J. Clin. Nutr 67(1), 12-17.
- 61.Mazloom Z, Hejazi N, M H Dabbaghmanesh, H R Tabatabaei, Ahmadi A et al. (2011) Effect of vitamin C supplementation on postprandial oxidative stress and lipid profile in type 2 diabetic patients. , Pak. J. Biol. Sci 14(19), 900-904.
- 62.Ghanwat Ganesh, Sontakke. (2019) Effect of Vitamin C Supplementation on Insulin Resistance, β-cell Function and Insulin Sensitivity in Obese and Non Obese Individuals. , Indian Journal of Public Health Research & Development 10, 10-5958.
- 63.Luo Xiaoqin. (2021) Dietary Vitamin C Intake Is Associated with Improved Liver Function and Glucose Metabolism in. , Chinese Adults.” Frontiers, Frontiers 29, 10-3389.
- 64.a Fl. (2020) . Effect of Vitamin C and E on Oxidative Stress and Antioxidant System in the Salivary Glands of STZ-Induced Diabetic Rats.” Archives of Oral Biology, Pergamon, 16 0003996920301436.
- 65.Toraman Ayşe. (2020) Effects of Vitamin C Local Application on Ligature-Induced Periodontitis in Diabetic Rats.”. , Journal of Applied Oral Science, Faculdade De Odontologia De Bauru - USP 27, 5-8.
- 66.Mohammed S Ellulu. (2015) Asmah Rahmat, Ismail Patimah, Huzwah Khaza’ai & Yehia Abed. 9, 3405-3412.
- 67.N H Gokhale, A B, V S Patil, D J Trivedi, S L Thakur. (2013) A short-term evaluation of the relationship between plasma ascorbic acid levels and periodontal disease in systemically healthy and type 2 diabetes mellitus subjects. , J. Diet. Suppl 10(2), 93-104.
- 68.Mazloom Z, Ekramzadeh M, Hejazi N. (2013) Efficacy of supplementary vitamins C and E on anxiety, depression and stress in type 2 diabetic patients: a randomized, single-blind, placebocontrolled trial. , Pak. J. Biol. Sci 16(22), 1597-1600.
- 69.Som S, Basu S, Mukherjee D, Deb S, Choudhury P R et al. (1981) Chatterjee IB: Ascorbic acid metabolism in diabetes mellitus. , Metabolism 30, 572-577.
- 70.Newill A, Habibzadeh N, Bishop N. (1984) Schorah CJ: Plasma levels of vitamin C components in normal and diabetic subjects. , Ann Clin Biochem 21, 488-490.
- 71.Stankova L, Riddle M, Burry K LarnedJ, Meneshe D, Hart J et al. (1984) Plasma ascorbate concentrations and blood cell dehydroascorbate transport in patients with diabetes mellitus. , Metabolism 33, 347-353.
- 72.Ginter E, Zdichynec B, Holzerova O, Ticha E, Kobza R et al. (1978) Hypocholesterolemic effect of ascorbic acid in maturity-onset diabetes mellitus. , Int J Vitam Nutr Res 48, 368-373.
- 73.Cox B D, Butterfield. (1975) WJH: Vitamin C supplements and diabetic cutaneous capillary fragility. , Br MedJ 3, 205.
- 74.Bishop N, Schorah C J, Wales.JK: The effect of vitamin C supplementation on diabetic hyperlipidemia: a double blind, crossover study. Diabetic Med 2:. 121-124.
- 77.S E Rajpathak, J P Crandall, Wylie-Rosett J, G C Kabat, T E Rohan et al.. The role of iron in type2 diabetes in human
- 78.Bao W, Rong Y, Liu L.Dietary iron intake, body iron stores, and the risk of type2 diabetes: a systemic review and meta-analysis.
- 79.Liu Q, Sum L, Tan Y, Wang G, Lin X et al.Role of iron deficiency and overload in the pathogenesis of diabetes and diabetic comlpicaion.
- 81.Fu X, S G Harshman, Shen X, D B Haytowitz, J P Karl et al.Multiple Vitamin K Forms Exist In Dairy Foods. , Curr. Dev. Nutr 2017, 10-3945.
- 82.Ibarrola-Jurado N, Salas-Salvadó J, Martínez-González M A And Bulló, M. (2012) Dietary phylloquinone intake and risk of type 2 diabetes in elderly subjects at high risk of cardiovascular disease. , Am. J. Clin. Nutr 96(5), 1113-1118.
- 83.J W Beulens, A D L van der, D E Grobbee, L Spijkerman Sluijs, M A et al. (2010) Dietary Phylloquinone and menaquinones intakes and risk of type 2 Diabetes. Diabetes Care. 33(8), 1699-1705.
- 84.Rees K, Guraewal S, Y L Wong, D L Majanbu, A Stranges Mavrodaris et al.vitamin K consumption associated with cardio-metabolicd isorders? A systematic review. , Maturitas 67(2), 121-128.
- 85.K B Comerford. (2013) Recent developments in multivitamin/mineral research. , Adv. Nutr 4(6), 644-656.
- 86.Ibarrola-Jurado N, Salas-Salvadó J, Martínez-González M A and Bulló, M. (2012) Dietary phylloquinone intake and risk of type 2 diabetes in elderly subjects at high risk of cardiovascular disease. , Am. J. Clin. Nutr 96(5), 1113-1118.
- 87.J W Beulens, A D L van der, D E Grobbee, Sluijs Spijkerman, M A et al. (2010) Dietary phylloquinone and menaquinones intakes and risk of type 2 diabetes. Diabetes Care. 33(8), 1699-1705.
- 88.Rees K, Guraewal S, Y L Wong, D L Majanbu, Mavrodaris A et al. (2010) Is vitamin K consumption associated with cardio-metabolic disorders? A systematic review. , Maturitas 67(2), 121-128.
- 89.K B Comerford. (2013) Recent developments in multivitamin/ mineral research. , Adv. Nutr 4(6), 644-656.
- 90.Rasouli N, I G Brodsky, Chatterjee R, S H Kim, R E Pratley et al.. D2d Research Group (2022). Effects of Vitamin D Supplementation on Insulin Sensitivity and Secretion in Prediabetes. The Journal of clinical endocrinology and metabolism 107(1), 230-240.
- 91.Mitri J, A G Pittas. (2014) Vitamin D and diabetes. Endocrinology and metabolism clinics of. , North America 43(1), 205-232.
- 92.Lips P, Eekhoff M, N van Schoor, Oosterwerff M, R de Jongh et al. (2017) Vitamin D and type 2 diabetes. The Journal of steroid biochemistry and molecular biology. 173-280.
- 93.Yu J, Sharma P, C M Girgis, J E Gunton. (2022) . Vitamin D and Beta Cells in Type 1 Diabetes: A Systematic Review. International journal of molecular sciences 23(22), 14434-10.
- 94.Manousaki D, Harroud A, R E Mitchell, Ross S, Forgetta V et al. (2021) Vitamin D levels and risk of type 1 diabetes: A Mendelian randomization study. PLoS medicine. 18(2), 1003536-10.
- 95.Zhang Y, Tan H, Tang J, Li J, Chong W et al. (2020) . Effects of Vitamin D Supplementation on Prevention of Type 2 Diabetes in Patients With Prediabetes: A Systematic Review and Meta-analysis. Diabetes care 43(7), 1650-1658.
- 96.Niroomand M, Fotouhi A, Irannejad N, Hosseinpanah F. (2019) Does high-dose vitamin D supplementation impact insulin resistance and risk of development of diabetes in patients with pre-diabetes? A double-blind randomized clinical trial. Diabetes research and clinical practice. 148-1.
- 97.A G Pittas, Kawahara T, Jorde R, Dawson-Hughes B, E M Vickery et al. (2023) . Vitamin D and Risk for Type 2 Diabetes in People With Prediabetes : A Systematic Review and Meta-analysis of Individual Participant Data From 3 Randomized Clinical Trials. Annals of internal medicine 176(3), 355-363.
- 98.Li X, Liu Y, Zheng Y, Wang P, Zhang Y. (2018) . The Effect of Vitamin D Supplementation on Glycemic Control in Type 2 Diabetes Patients: A Systematic Review and Meta-Analysis. Nutrients 10(3), 375-10.
- 99.Cojic M, Kocic R, Klisic A, Kocic G. (2021) . The Effects of Vitamin D Supplementation on Metabolic and Oxidative Stress Markers in Patients With Type 2 Diabetes: A 6-Month Follow Up Randomized Controlled Study. Frontiers in endocrinology 12, 610893-10.
- 100.Mitri J, Pittas A G. (2014) Vitamin D and diabetes. , Endocrinol. Metab. Clin. North. Am 43(1), 205-32.
- 101.Mathieu C, Gysemans C, Giulietti A, Bouillon R. (2005) Vitamin D and diabetes. , Diabetologia 48(7), 1247-1257.
- 102.Ghavamzadeh S, Mobasseri M, Mahdavi R. (2014) The Effect of Vitamin D Supplementation on Adiposity, Blood Glycated Hemoglobin, Serum Leptin and Tumor Necrosis Factor-α in Type 2 Diabetic Patients. , Int. J. Prev. Med 5(9), 1091-1098.
- 103.T R Neyestani, Nikooyeh B, Alavi-Majd H, Shariatzadeh N, Kalayi A et al. (2012) Improvement of vitamin D status via daily intake of fortified yogurt drink either with or without extra calcium ameliorates systemic inflammatory biomarkers, including adipokines, in subjects with type 2 diabetes. , J. Clin. Endocrinol. Metab 97(6), 2001-2011.
- 104.Witham Dove, F J Dryburgh, Sugden M, J A Morris, D A et al. (2010) The effect of different doses of vitamin D3 on markers of vascular health in patients with type 2 diabetes: a randomized controlled trial. , Diabetologia 53, 21122119.
- 105.O H Ryu, Lee S, Yu J, M G Choi, H J Yoo et al. (2014) A prospective randomized controlled trial of the effects of vitamin D supplementation on long-term glycemic control in type 2 diabetes mellitus of Korea. , Endocr. J 61(2), 167-176.
- 106.K M Alkharfy, N M Al-Daghri, S B, Al-Othman A, Moharram O et al. (2013) Vitamin D supplementation in patients with diabetes mellitus type 2 on different therapeutic regimens: a oneyear prospective study. , Cardiovasc. Diabetol 12(1), 113.
- 107.Nilas L, Christiansen C. (1984) Treatment with vitamin D or its analogues does not change body weight or blood glucose level in postmenopausal women. , Int. J. Obes 8(5), 407-411.
- 108.J A Sugden, Davie s J I, Witham Morris, D A, A D Struthers. (2008) Vitamin D improves endothelial function in patients with Type 2 diabetes mellitus and low vitamin D levels. , Diabet. Med 25(3), 320-325.
- 109.Jorde R, Figenschau Y. (2009) Supplementation with cholecalciferol does not improve glycaemic control in diabetic subjects with normal serum 25-hydroxyvitamin D levels. , Eur. J. Nutr 6, 349-354.
- 110.E Van, Mathieu C. (2005) Immunoregulation by 1,25dihydroxyvitamin D3: basic concepts. , J. Steroid Biochem. Mol. Biol 97(1), 93-101.
- 111.Brusgaard L, Christesen K, H T Schuit, Proost F, Masini P et al. (2014) Discovery of molecular pathways mediating dihydroxyvitamin D3 protection against 1,25cytokine-induced inflammation and damage of human and male mouse islets of Langerhans. , Endocrinology 155(3), 736-747.
- 112.Kayaniyil S, Vieth R, Retnakaran R, J A Knight, Qi Y et al. (2010) Association of vitamin D with insulin resistance and beta-cell dysfunction in subjects at Diabetes Care. 33(6), 1379-1381.
- 113.A R Broder, J N Tobin, Putterman C.Disease-specific definitions of vitamin D deficiency need to be established in autoimmune and non-autoimmune chronic diseases: a retrospective comparison of three chronic diseases. Arthritis Res. , Ther 12(5), 191.
- 114.Bellan M, Guzzaloni G, Rinaldi M, Merlotti E, Ferrari C et al.and Marzullo P.(2014) Altered glucose metabolism rather than naive type 2 diabetes mellitus (T2DM) is related to vitamin D status in severe obesity. , Cardiovascular Diabetology 13, 57.
- 115.Kostoglou-Athanassiou I, Athanassiou P, Gkountouvas A, Kaldrymides P. (2013) Vitamin D and glycemic control in diabetes mellitus type 2. , Ther. Adv. Endocrinol. Metab 4(4), 122-8.
- 116.Stadlmayr A, Aigner E, Huber-Schönauer U, Niederseer D, Zwerina J et al. (2014) Relations of vitamin D status, gender and type 2 diabetes in middle-aged Caucasians. Acta Diabetol. [Epub ahead of print].
- 117.Frank L L. (2015) Thiamin in Clinical Practice. Epub , JPEN J Parenter Enteral Nutr 39(5), 503-20.
- 118.Hershkowitz E, Markel A. (2015) THIAMINE--"THE ROAD EXPERIENCE". Oct;154(10): 661-4, 674. Hebrew. PMID: , OF THE VITAMIN AS A MANIFESTATION OF DEFICIENCY IN A WORLD OF ABUNDANCE]. Harefuah 26742231.
- 119.Ishiko T, Taguchi T, Takeguchi M, Saito H, Nanri K. (2009) [Case of Wernicke's encephalopathy and subacute combined degeneration of the spinal cord due to vitamin deficiency showing changes in the bilateral corpus striatum and cardiac arrest due to beriberi heart disease]. Brain Nerve. , Japanese. PMID: 61(9), 1069-73.
- 120.Ziegler Dan. (2023) Association between diabetes and thiamine status - A systematic review and meta-analysis.” Metabolism: clinical and experimental vol. 144, 155565-10.
- 121.Muley A, Fernandez R, Green H.Effect of thiamine supplementation on glycaemic outcomes in adults with type 2 diabetes: a systematic review and meta-analysis. , BMJ Open 2022, 10-113.
- 122.Structure.mechanism and catalytic duality of thiamine-dependent enzymes. , Cell Mol Life Sci 2007, 892-905.
- 123.Ge Yanyan. (2023) Pregnancy thiamine and riboflavin intake and the risk of gestational diabetes mellitus: A prospective cohort study.” The American journal of clinical nutrition. 117, 426-435.
- 124.Beltramo Elena. (2021) Thiamine and diabetes: back to the future?.”. , Acta diabetologica 58, 1433-1439.
- 125.Oh S H, Witek R P, Bae S H, Darwiche H, Jung Y et al. (2009) Detection of transketolase in bone marrow-derived insulin-producing cells: benfotiamine enhances insulin synthesis and glucose metabolism. Stem Cells Dev. Jan-Feb;18(1): 37-46. doi: 10.1089/scd.2007.0255. PMID: 18393672; PMCID: PMC3118870 .
- 126.Hammes H-P, Edelstein D DuX.Benfotiamine blocks three major pathways of hyperglycemic damage and prevents experimental diabetic retinopathy.NatMed2003;9:. 294-9.
- 127.Page G L J. (2011) Thiamine deficiency in diabetes mellitus and the impact of thiamine replacement on glucose metabolism and vascular disease.”. , International journal of clinical practice 65, 684-90.
- 128.Hobara R, Ozawa K, Okazaki M, Yasuhara H. (1981) Relationship between thiamine and glucose levels in diabetes mellitus. , J Pharmacol 31, 1098-1100.
- 129.Kjosen M S. (1977) Seim SH: The transketolase assay of thiamine in some diseases. , Am] Clin Nutr 30, 1591-1596.
- 130.Lewis C M, Canafax D M, Sprafka J M, Barbosa J J. (1992) Double-blind randomized trial of nicotinamide on early-onset diabetes. , Diabetes Care 15, 121-123.
- 131.O S Al-Attas, N M Al-Daghri, A, S H Abd-Alrahman, Sabico S. (2012) Blood thiamine and its phosphate esters as measured by high-performance liquid chromatography: levels and associations in diabetes mellitus patients with varying degrees of microalbuminuria. , J. Endocrinol. Invest 35(11), 951-956.
- 132.Waheed P, A K Naveed, Ahmed T. (2013) Thiamine deficiency and its correlation with dyslipiaemia in diabetics with microalbuminuria. , J. Pak. Med. Assoc 63(3), 340-345.
- 133.González-Ortiz M, Martínez-Abundis E, J A Robles-Cervantes, Ramírez-Ramírez V, M G Ramos-Zavala. (2010) Effect of thiamine administration on metabolic profile, cytokines and inflammatory markers in drug-naïve patients with type 2 diabetes. , Eur. J. Nutr 50(2), 145-149.
- 134.Rabbani N, S, Riaz S, J R Larkin, M W Akhtar et al. (2009) High-dose thiamine therapy for patients with type 2 diabetes and microalbuminuria: randomised, double-blind placebo-controlled pilot Diabetologia. 52(2), 208-212.
- 135.Xiang Dan. (2020) Effectiveness of niacin supplementation for patients with type 2. , diabetes.” Medicine 99, 10-1097.
- 136.D’Andrea Elvira.Assessment of the role of niacin in managing cardiovascular disease outcomes.”. , JAMA Network Open 2, 2019-10.
- 137.Tavintharan Subramaniam. (2009) Effects of niacin on cell adhesion and early atherogenesis: Biochemical and functional findings in endothelial cells.” Basic &. , Clinical Pharmacology & Toxicology 104, 206-210.
- 138.Landau C. (1992) Effect of nicotinic acid on plasma glucose concentration in normal individuals.”. , Hormone and Metabolic Research 24, 424-428.
- 139.Sazonov V, Maccubbin D, C M Sisk, P L Canner. (2013) Effects of niacin on the incidence of new onset diabetes and cardiovascular events in patients with normoglycaemia impaired fasting glucose. , Int. J. Clin. Pract 67(4), 297-302.
- 140.Merigliano C, Mascolo E, Burla R, Saggio I, Vernì F.The relationship between vitamin B6, diabetes and cancer. Frontiers in Genetics. 2018, 388.
- 141.Mascolo E, Vernì F.Vitamin B6 and diabetes: relationship and molecular mechanisms. International journal of molecular sciences. 21(10), 3669.
- 142.Okada M, Shibuya M, Yamamoto E, Murakami Y.Effect of diabetes on vitamin B6 requirement in experimental animals. , Diabetes, Obesity and Metabolism 1(4), 221-5.
- 143.Jain S K.Vitamin B6 (pyridoxamine) supplementation and complications of diabetes. , Metabolism-Clinical and Experimental 56(2), 168-71.
- 145.Tanimoto M, Gohda T, Kaneko S, Hagiwara S, Murakoshi M et al.Effect of pyridoxamine (K-163), an inhibitor of advanced glycation end products, on type 2 diabetic nephropathy in KK-Ay/Ta mice. , Metabolism 56(2), 160-7.
- 146.Ellis J M, Folkers K, Minadeo M, VanBuskirk R, Xia L-J et al.A deficiency of vitamin B6 is a plausible molecular basis of the retinopathy of patients with diabetes mellitus. Biochemical and biophysical research communications. 179(1), 615-9.
- 147.Satyanarayana A, Balakrishna N, Pitla S, Reddy P Y, Mudili S et al.Status of B-vitamins and homocysteine in diabetic retinopathy: association with vitamin-B12 deficiency and hyperhomocysteinemia. , PloS one 6(11).
- 148.Ahn H J, Min K W, Cho Y-O.Assessment of vitamin B6 status in Korean patients with newly diagnosed type 2 diabetes. Nutrition research and practice. 5(1), 34-9.
- 149.Nix W A, Zirwes R, Bangert V, Kaiser R P, Schilling M et al.Vitamin B status in patients with type 2 diabetes mellitus with and without incipient nephropathy. Diabetes research and clinical practice. 107(1), 157-65.
- 150.Bennink H, Schreurs W.Improvement of oral glucose tolerance in gestational diabetes by pyridoxine. , Br Med J 3(5974), 13-5.
- 151.Nair A, Biju M, Paulose C.Effect of pyridoxine and insulin administration on brain glutamate dehydrogenase activity and blood glucose control in streptozotocin-induced diabetic rats. , Biochimica et Biophysica Acta (BBA)-General Subjects 1381(3), 351-4.
- 152.Solomon L R, Cohen K.Erythrocyte O2 transport and metabolism and effects of vitamin B6 therapy in type II diabetes mellitus. , Diabetes 38(7), 881-6.
- 153.Kim H H, Kang Y-R, Lee J-Y, Chang H-B, Lee K W et al.The postprandial anti-hyperglycemic effect of pyridoxine and its derivatives using in vitro and in vivo animal models. , Nutrients 10(3), 285.
- 154.Spellacy W, Buhi W, Birk S.Vitamin B6 treatment of gestational diabetes mellitus: studies of blood glucose and plasma insulin. American journal of obstetrics and gynecology. 127(6), 599-602.
- 155.Zhu J, Chen C, Lu L, Yang K, Reis J et al.Intakes of folate, vitamin B6, and vitamin B12 in relation to diabetes incidence among American young adults: a 30-year follow-up study. Diabetes Care. 43(10), 2426-34.
- 156.Asbaghi O, Ashtary-Larky D, Bagheri R, S P Moosavian, H P Olyaei et al. (2021) Folic Acid Supplementation Improves Glycemic Control for Diabetes Prevention and Management: A Systematic Review and Dose-Response Meta-Analysis of Randomized Controlled Trials. , Nutrients 13, 2355-10.
- 157.Lin L, Du Y, Niu G. (2023) Folate deficiency may increase the risk for elevated TSH in patients with type 2 diabetes mellitus. , BMC Endocr Disord 23, 169-10.
- 158.Liu W, Cao S, Shi D, Ye Z, Yu L et al. (2023) Daily folate consumption is associated with reduced all-cause and cardiovascular disease mortality among US adults with diabetes, prediabetes, or insulin resistance. , Nutrition Research 114, 71-80.
- 159.Zhao J, Schooling C, Zhao J. (2018) The effects of folate supplementation on glucose metabolism and risk of type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. , Annals of Epidemiology 28(4), 249-257.
- 160.Zhao M, M, Yuan L, L, J H Liao et al. (2018) Chronic folate deficiency induces glucose and lipid metabolism disorders and subsequent cognitive dysfunction in mice. , PloS one 13(8), 0202910-10.
- 161.Catherine M Clase, Ki Vincent, Rachel M Holden. (2013) Water‐soluble vitamins in people with low glomerular filtration rate or on dialysis: a review.”. In Seminars in dialysis 26, 546-567.
- 162.Takahashi Yoshiastu. (1994) Shinichirou Takayama, Takeshi Itou, Kazuhiro Owada, and Yasue Omori. “Effect of glycemic control on vitamin. B12 metabolism in diabetes mellitus.” Diabetes research and clinical practice 25 1, 13-17.
- 163.Satapathy Swayamjeet, Bandyopadhyay Debapriya, Binod Kumar Patro, Khan Shahnawaz, Naik Sanjukta. (2020) Folic acid and vitamin B12 supplementation in subjects with type 2 diabetes mellitus: A multi-arm randomized controlled clinical trial.” Complementary therapies in medicine 53. 102526.
- 164.Jun S Lai, Wei, Cai Shirong, Yung Seng Lee, Jerry KY Chan. (2018) Lynette PC Shek, Fabian KP Yap et al. “High folate and low vitamin B12 status during pregnancy is associated with gestational diabetes mellitus.”. , Clinical nutrition 37, 940-947.
- 165.Kouroglou Eleni. (2019) Panagiotis Anagnostis, Alexandros Daponte, and Alexandra Bargiota. “Vitamin B12 insufficiency is associated with increased risk of gestational diabetes mellitus: a systematic review and meta-analysis.”. , Endocrine 66, 149-156.
- 166.Kos Elizabeth, Mary Jo Liszek, Mary Ann Emanuele, Durazo-Arvizu Ramon, Camacho Pauline. (2012) . Effect of metformin therapy on vitamin D and vitamin B12 levels in patients with type 2 diabetes mellitus.” Endocrine Practice 18 2, 179-184.
- 167.Valdés-Ramos Roxana, Guadarrama-Lopez Ana Laura. (2015) Martinez-Carrillo Beatriz Elina, and Benitez-Arciniega Alejandra Donaji. “Vitamins and type 2 diabetes mellitus.”. , Endocrine, Metabolic & Immune Disorders-Drug Targets (Formerly Current Drug Targets-Immune, Endocrine & Metabolic Disorders) 15, 54-63.
- 168.Badedi Mohammed, Darraj Hussain, Hummadi Abdulrahman, Solan Yahia, Zakri Ibrahim et al. (2019) Vitamin b12 deficiency and foot ulcers in type 2 diabetes mellitus: A case–control study.” Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy. 2589-2596.
- 169.Kibirige Davis, Mwebaze Raymond. (2013) Vitamin B12 deficiency among patients with diabetes mellitus: is routine screening and supplementation justified?.”. , Journal of Diabetes & Metabolic Disorders 12, 1-6.
- 170.Xiong Shuyuan, Liu Zhiping, Yao Ning, Zhang Xiaoru, Ge Qian. (2022) The independent association between vitamin B12 and insomnia in Chinese patients with type 2 diabetes mellitus: a cross-sectional study.”. , Nutrition & Diabetes 12, 3.
- 171.Shaun A Mason. (2021) Effects of Vitamin C Supplementation on Glycemic Control and Cardiovascular Risk Factors in People with Type 2 Diabetes: A Grade-Assessed Systematic Review and. , Meta-Analysis of Randomized Controlled Trials.” American Diabetes Association, American Diabetes Association 13, 44-2.
- 172.a S. (2019) . Impact of Rutin and Vitamin C Combination on Oxidative Stress and Glycemic Control in Patients with Type 2 Diabetes.” Clinical Nutrition ESPEN, Elsevier 14, 2405457719304887.
- 173.a Fl. (2020) . Effect of Vitamin C and E on Oxidative Stress and Antioxidant System in the Salivary Glands of STZ-Induced Diabetic Rats.” Archives of Oral Biology, Pergamon, 16 0003996920301436.
- 174.Toraman Ayşe. (2020) Effects of Vitamin C Local Application on Ligature-Induced Periodontitis in Diabetic Rats.”. , Journal of Applied Oral Science, Faculdade De Odontologia De Bauru - USP 27, 5-8.
- 175.Mohammed S Ellulu. (2015) Asmah Rahmat, Ismail Patimah, Huzwah Khaza’ai & Yehia Abed. 9, 3405-3412.
- 176.Oldenburg J, Marinova M, Müller-Reible C, Watzka M. (2008) The vitamin K cycle. , Vitam Horm 78, 35-62.
- 177.Hussein A G, Mohamed R H, Shalaby S M.Abd El Motteleb DM (2018) Vitamin K2 alleviates type 2 diabetes in rats by induction of osteocalcin gene expression. , Nutrition 47, 33-38.
- 178.Dihingia A, Ozah D, Ghosh S, Sarkar A, Baruah P K et al. (2018) Vitamin K1 inversely correlates with glycemia and insulin resistance in patients with type 2 diabetes (T2D) and positively regulates SIRT1/AMPK pathway of glucose metabolism in liver of T2D mice and hepatocytes cultured in high glucose. , J Nutr Biochem 52, 103-114.
- 179.Rasekhi H, Karandish M, Jalali M T. (2015) Phylloquinone supplementation improves glycemic status independent of the effects of adiponectin levels in premenopause women with prediabetes: A double-blind randomized controlled clinical trial. J Diabetes Metab Disord. 14, 1.
- 180.Ferrannini E, Natali A, Bell P, Cavallo-Perin P, Lalic N et al. (1997) Insulin resistance and hypersecretion in obesity:. , European Group for the Study of Insulin Resistance (EGIR). J Clin Invest 100, 1166-1173.
- 181.Yoshida M, Booth S, Meigs J, Saltzman E, Jacques P. (2008) Phylloquinone intake, insulin sensitivity, and glycemic status in adult men and women. , Am J Clin Nutr88: 210.
- 182.Cockayne S, Adamson J, Lanham-New S, Shearer M J, Gilbody S et al. (2006) Vitamin K and the prevention of fractures: systematic review and meta-analysis of randomized controlled trials. , Arch Intern Med 166(12), 1256-1261.
- 183.Torgerson D J. (2018) Caution to readers about systematic review on vitamin K and prevention of fractures that included problematic trials. , JAMA Intern Med 178(6), 863-864.
- 184.Chukwuma C I. (2020) Zinc(II) complexes in diabetes management: Plant-derived phenolics as understudied promising ligands. J Food Biochem. Nov;44(11):e13477. doi: 10.1111/jfbc.13477. Epub 32984986.
- 185.Vardatsikos G, Pandey N R, Srivastava A K. (2013) Insulino-mimetic and anti-diabetic effects of zinc. J Inorg Biochem. Mar;120: 8-17. doi: 10.1016/j.jinorgbio.2012.11.006. Epub 2012 Dec 3. PMID: 23266931.
- 186.Jayawardena R, Ranasinghe P, Galappatthy P, Malkanthi R, Constantine G et al. (2012) Effects of zinc supplementation on diabetes mellitus: a systematic review and meta-analysis. Diabetol Metab Syndr. 13-10.
- 187.Barman S, Srinivasan K. (2020) Diabetes and zinc dyshomeostasis: Can zinc supplementation mitigate diabetic complications?. Epub , Crit Rev Food Sci Nutr 62(4), 1046-1061.
- 188.Chukwuma C I, Mashele S S, Eze K C, Matowane G R, Islam S M et al. (2020) A comprehensive review on zinc(II) complexes as anti-diabetic agents: The advances, scientific gaps and prospects. Pharmacol Res. May;155: 104744. doi: 10.1016/j.phrs.2020.104744. Epub 2020 Mar 7. PMID: 32156651.
- 189.Ghaedi K, Ghasempour D, Jowshan M, Zheng M, Ghobadi S et al. (2023) Effect of zinc supplementation in the management of type 2 diabetes: A grading of recommendations assessment, development, and evaluation-assessed, dose-response meta-analysis of randomized controlled trials. Crit Rev Food Sci Nutr. Epub ahead of print. PMID: 15, 1-12.
- 190.Wang X, Wu W, Zheng W, Fang X, Chen L et al. (2019) Zinc supplementation improves glycemic control for diabetes prevention and management: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr. 110(1), 76-90.
- 191.Bjørklund G, Dadar M, Pivina L, Doşa M D, Semenova Y et al.The Role of Zinc and. Copper in Insulin Resistance and Diabetes Mellitus. Curr Med Chem 27(39), 6643-6657.
- 192.Zheng Y, Li X K, Wang Y, Cai L.The role of zinc, copper and iron in the pathogenesis of diabetes and diabetic complications: therapeutic effects by chelators. , Hemoglobin 32(1), 135-45.
- 193.Landau C. (1992) Effect of nicotinic acid on plasma glucose concentration in normal individuals.”. , Hormone and Metabolic Research 24, 424-428.
- 194.Muley A, Fernandez R, Green H.Effect of thiamine supplementation on glycaemic outcomes in adults with type 2 diabetes: a systematic review and meta-analysis. , BMJ Open 2022, 10-113.
- 195.Ge Yanyan. (2023) Pregnancy thiamine and riboflavin intake and the risk of gestational diabetes mellitus: A prospective cohort study.” The American journal of clinical nutrition. 117, 426-435.
- 196.raber M G, Jialal I. (2013) Measurement of lipid-soluble vitamins-a further adjustment needed? Lancet 2000;355:. 55.
- 197.Thurnam D I, Davies J A, Cramp B J, Situnayake R D, Davis M.The use of different lipids to express serum tocopherol: lipid ratios of the measurement of vitamin E status. , Ann Clin Biochem 1986, 51.
- 198.Shin S M, Razdan B, Mishra O P, Johnson L, Delivoriapapadopoulos M.Protective effect of -tocopherol on brain cell membrane function during cerebral cortical hypoxia in newborn piglets. , Brain Res 1994, 45.
- 199.Kay P, Tesh R, Woo J, Chin D, Muk Y T. (1989) Serum vitamin E concentration in adult-onset spinocerebellar degeneration. , J Neurol Neurosurg Psychiatry 52-131.
- 200.Farbstein D, Blum S, Pollak M, Asaf R, H L Viener et al. (2011) Vitamin E therapy results in a reduction in HDL function in individuals with diabetes and the haptoglobin 2-1 genotype. , Atherosclerosis 219(1), 240-244.
- 201.Vatassery G T, Morley H E. (1983) Kuskowski MA: Vitamin E in plasma and platelets of human diabetic patients and control subjects. , Am J Clin Nutr 37, 641-644.
- 202.Asbaghi O, Sadeghian M.Efects of zinc supplementation on lipid profle in patients with type 2 diabetes mellitus: A systematic review and meta-analysis of randomized controlled trials. Nutr Metab Cardio vasc Dis. 30(8), 1260-71.
- 203.GCJ Tan, SMQ Tan.Tocotrienol-rich vitamin E improves dia betic nephropathy and persists 6–9 months after washout: a phase IIa randomized controlled trial. Ther Adv Endocrinol Metab. 2019, 2042018819895462-10.
- 204.Xu R, Zhang S.Infuence of vitamin E supplementation on glycae mic control: a meta-analysis of randomised controlled trials. PLoS One. 9(4), 10-1371.
- 205.Blum S, Vardi M, J B Brown, Russell A, Milman U et al. (2010) Vitamin E reduces cardiovascular disease in individuals with diabetes mellitus and the haptoglobin 2-2 genotype. , Pharmacogenomics 11(5), 675-684.
- 206.Li F, Pei L. () Infuence of omega -3 fatty acid and vitamin co -supple mentation on metabolic status in gestational diabetes: A meta -analysis of randomized controlled studies. https://doi.org/10.1016/j.ejogrb.2020.02.024 , Eur J Obstet Gynecol Reprod Biol 2020.
- 207.C F Haskell, Robertson B, Jones E, Forster J, Jones R et al. (2010) Effects of a multi-vitamin/mineral supplement on cognitive function and fatigue during extended multi-tasking. Available from: https://doi.org/10.1002/hup.1144 , Hum. Psychopharmacol 25, 448-461.
- 208.L H Allen. (2008) Causes of vitamin B12 and folate deficiency. , Food Nutr. Bull. 29,. Available from:, 10-1177.
- 209.L H Allen. (2009) How common is vitamin B-12 deficiency?. Available from: https://doi.org/10.3945/ajcn.2008.26947A , Am. J. Clin. Nutr 89, 693-696.
- 210.Dali-Youcef N, Andrès E. (2009) An update on cobalamin deficiency in adults. Available from: https://doi.org/10.1093/qjmed/hcn138 , QJM 102, 17-28.
- 211.R G Deeley, Westlake C, Cole S P C. (2006) Transmembrane transport of endo- and xenobiotics by mammalian ATP-binding cassette multidrug resistance proteins. Available from: https://doi.org/10.1152/physrev.00035.2005 , Physiol. Rev 86, 849-899.
- 212.Coelho D, Suormala T, Stucki M, J P Lerner-Ellis, D S Rosenblatt et al. (2008) Gene identification for the cblD defect of vitamin B12 metabolism. Available from: https://doi.org/10.1056/ NEJMoa072200 , N. Engl. J. Med 358, 1454-1464.
- 213.Institute of Medicine (1998) Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline, The National Academies Collection: Reports funded by National Institutes of Health. , Washington, DC
- 214.Satyanarayana A, Balakrishna N, Pitla S, Reddy P Y, Mudili S et al.Status of B-vitamins and homocysteine in diabetic retinopathy: association with vitamin-B12 deficiency and hyperhomocysteinemia. , PloS one 6(11).
- 215.Ahn H J, Min K W, Cho Y-O.Assessment of vitamin B6 status in Korean patients with newly diagnosed type 2 diabetes. Nutrition research and practice. 5(1), 34-9.
- 216.Nix W A, Zirwes R, Bangert V, Kaiser R P, Schilling M et al.Vitamin B status in patients with type 2 diabetes mellitus with and without incipient nephropathy. Diabetes research and clinical practice. 107(1), 157-65.
- 217.Bennink H, Schreurs W.Improvement of oral glucose tolerance in gestational diabetes by pyridoxine. , Br Med J 3(5974), 13-5.
- 218.Nair A, Biju M, Paulose C.Effect of pyridoxine and insulin administration on brain glutamate dehydrogenase activity and blood glucose control in streptozotocin-induced diabetic rats. , Biochimica et Biophysica Acta (BBA)-General Subjects 1381(3), 351-4.
- 219.Solomon L R, Cohen K.Erythrocyte O2 transport and metabolism and effects of vitamin B6 therapy in type II diabetes mellitus. , Diabetes 38(7), 881-6.
- 220.Kim H H, Kang Y-R, Lee J-Y, Chang H-B, Lee K W et al.The postprandial anti-hyperglycemic effect of pyridoxine and its derivatives using in vitro and in vivo animal models. , Nutrients 10(3), 285.
- 221.Spellacy W, Buhi W, Birk S.Vitamin B6 treatment of gestational diabetes mellitus: studies of blood glucose and plasma insulin. American journal of obstetrics and gynecology. 127(6), 599-602.
- 222.Zhu J, Chen C, Lu L, Yang K, Reis J et al.Intakes of folate, vitamin B6, and vitamin B12 in relation to diabetes incidence among American young adults: a 30-year follow-up study. Diabetes Care. 43(10), 2426-34.
- 223.Asbaghi O, Ashtary-Larky D, Bagheri R, S P Moosavian, H P Olyaei et al. (2021) Folic Acid Supplementation Improves Glycemic Control for Diabetes Prevention and Management: A Systematic Review and Dose-Response Meta-Analysis of Randomized Controlled Trials. , Nutrients 13, 2355-10.
- 224.Lin L, Du Y, Niu G. (2023) Folate deficiency may increase the risk for elevated TSH in patients with type 2 diabetes mellitus. , BMC Endocr Disord 23, 169-10.
- 225.Liu W, Cao S, Shi D, Ye Z, Yu L et al. (2023) Daily folate consumption is associated with reduced all-cause and cardiovascular disease mortality among US adults with diabetes, prediabetes, or insulin resistance. , Nutrition Research 114, 71-80.
- 226.Zhao J, Schooling C, Zhao J. (2018) The effects of folate supplementation on glucose metabolism and risk of type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. , Annals of Epidemiology 28(4), 249-257.
- 227.Zhao M, M, Yuan L, L, J H Liao et al. (2018) Chronic folate deficiency induces glucose and lipid metabolism disorders and subsequent cognitive dysfunction in mice. , PloS one 13(8), 0202910-10.
- 228.Mitri J, A G Pittas. (2014) Vitamin D and diabetes. Endocrinology and metabolism clinics of. , North America 43(1), 205-232.
- 229.Yu J, Sharma P, C M Girgis, J E Gunton. (2022) . Vitamin D and Beta Cells in Type 1 Diabetes: A Systematic Review. International journal of molecular sciences 23(22), 14434-10.
- 230.Hou Y, Song A, Jin Y, Xia Q, Song G et al. (2021) A dose-response meta-analysis between serum concentration of 25-hydroxy vitamin D and risk of type 1 diabetes mellitus. European journal of clinical nutrition. 75(7), 10-1038.
- 231.Bartley J. (2010) Vitamin D: emerging roles in infection and immunity. Expert review of anti-infective therapy. 8(12), 1359-1369.
- 232.Niroomand M, Fotouhi A, Irannejad N, Hosseinpanah F. (2019) Does high-dose vitamin D supplementation impact insulin resistance and risk of development of diabetes in patients with pre-diabetes? A double-blind randomized clinical trial. Diabetes research and clinical practice. 148-1.