Construction of Virtual Neuron and Consolidation of Sleep and Memory Process– A Molecular Docking and Biomathematical Approach
Abstract
This methods paper combines molecular docking and biomathematical modeling to construct a virtual neuron framework for studying sleep‑related memory consolidation. It outlines model components and validation approach.
Author Contributions
Academic Editor: Fengji Luo, School of Electrical Engineering and Telecommunications, University of New South Wales, Australia.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2019 Bin Zhao, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
We have no conflict of interests to disclose and the manuscript has been read and approved by all named authors.
Citation:
Introduction
The relationship between sleep and memory never fails to fascinate human beings as we always keep curious about how the memory form and consolidate 1. Nevertheless, recent developments in molecular genetics, neurophysiology, and the cognitive neurosciences have produced a striking body of research that provides converging evidence for an important role of sleep in learning and the reprocessing of memories.Also, sleep deprivation has become a prevalent public health epidemic with a wide range of harmful consequences, including memory and cognitive impairment 2, some scientists tend to focus attention on the neurobiological root of this universal human experience.
Previous Work
Effects of sleep stage on consolidation early study of different sleep stages in memory consolidation in rats mainly focused on REM sleep (Random Eye Movement sleep) and the consequences of REMD (REM sleep deprivation) by repeatedly waking subjects at the first signs of REM sleep. All these memory tasks were typically emotionally loaded as it has been proved that REM sleep priority to improve the consolidation of memory emotions3, 4, and it turned out that REMD is only valid for a specific period of time after learning — the so-called "REM sleep window"5, 6.
The first evidence of the causal role of SWS (Slow Wave Sleep) reactivation during memory consolidation comes from the study of human spatial location in the presence of odor7. Reactivation activates hippocampal memory redistribution to new cortical storage locations. On the other hand, another hypothesis, named ‘sequential hypothesis’, which argues that the best benefit of sleep for consolidating declarative and non-declarative memory occurs when SWS and REM sleep occur one after the other is also verified by the cross effect of SWS/REM on declarative/procedural memory consolidation8, 9.
The Effects of Sleep/sleep Deprivation on Memory
Sleep’s Promotion on Memory Consolidation
Sleep is characterized by the rapid occurrence of REM sleep and non-REM sleep, including slow wave sleep (SWS, Phases 3 and 4) and light sleep Phases 1 and 2 (see Figure 1, part a). . In
Figure 1.Sleep structure and Electrophysiological sleep stage characteristics
humans, the first part of the night (early sleep) is characterized by a high amount of SWS, while REM sleep is dominant in the lower half (late sleep). SWS and REM sleep are characterized by a specific pattern of electric field potential oscillations (see Figure 1, part
In subsequent rapid eye movement (REM) sleep, the brain system operates in a "separated" mode, which is also associated with separation between long-term and temporary storage. This enables a synaptic consolidation of the local encapsulation process, which reinforces the memory characterization of system integration during previous SWS (thick line). In general, memory gets the best results from slow wave sleep and rapid eye movement sleep. However, due to their different characteristics, integrated declarative memory (which combines the characteristics of different memories in different memory systems) theoretically benefits more from SWS-related system integration, with specific and discrete procedural memory benefits more from REM sleep associated synapse consolidation in the local brain circuit10. (Figure 2).
Figure 2.Sequential contributions of SWS and REM sleep on memory consolidation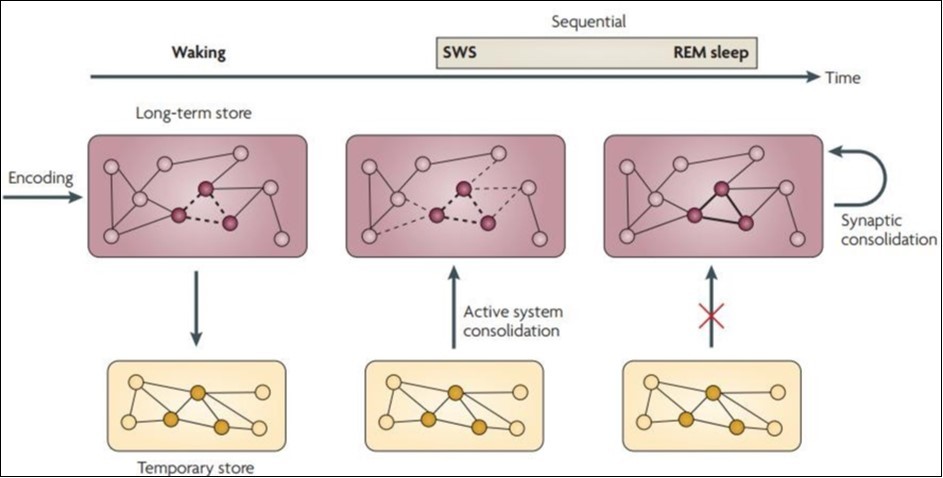
There are currently two hypotheses that explain the mechanism of memory consolidation during sleep. Synaptic homeostasis hypothesis11 is a by-product of the overall synaptic reduction that occurs during sleep. The active system consolidation hypothesis suggests that the active consolidation process is caused by selective reactivation of memory in sleep12. The former use is to serve a global downscale of synaptic strength by the slow oscillations to achieve sustainable levels in energy and tissue volume requirements and allow for the repetitive use of synapses in future coding, and the process of the latter can be depicted 13, 14. Therefor, we have the two-stage model and sequential contributions of SWS and REM sleep on memory consolidation (Figure 3).
Memory Impairment After Sleep Deprivation
Marion and his colleagues once used electrophysiological, molecular and behavioral indices to non-invasively study LTP-like plasticity in humans after sleep and sleep deprivation. The outcome shows that LTP-like plasticity decreases after sleep deprivation verified by declined MEP amplitudes, lower potential modulator level and less correctly recalled word-pairs during the declarative memory task15.
For further studying, Tudor et al extended that 5 hours of sleep loss attenuated both mTORC1-mediated phosphorylation of 4EBP2 and the interaction between eukaryotic initiation factor 4E (eIF4E) and eIF4G in the hippocampi of sleep-deprived mice using an in vivo protein translation assay. What is more, by increasing the abundance of 4EBP2 in hippocampal excitatory neurons before sleep deprivation the abundance of phosphorylated 4EBP2 increased, and the
amount of eIF4E-eIF4G interaction restored and hippocampal protein synthesis to that seen in mice that were not sleep-deprived, and prevented the hippocampus-dependent memory deficits associated with sleep loss16. Effects of sleep deprivation on protein synthesis procedure is also verified 17. (Figure 4)
Figure 4.Effects of sleep deprivation on protein synthesis procedure.
And the molecular neurobiology mechanism of sleep on protein synthesis procedure is also verified 18.
Next, we consider the neuronal functions in sleep and memory consolidation process by the following fractional-order differential equations:
Where 0 < ai< 1, (i= 1, 2, 3, 4) is a parameter describing the order of the system in sleep and memory consolidation process, xi (i= 1,2,3,4) is the anti-synchronization function of the time a= 35, b= 3, c= 12, h= 7,0.085 £ r£ 0.798 , then the system is in a chaotic state. Suppose that some ractional-order differential equations are response systems of the neuronal functions in sleep and memory consolidation process :
Where aˆ, bˆ, cˆ, hˆ, rˆ are the estimated values of the parameters a, b, c, h, r on the system (3.1) respectively; and 0 < bi< 1, (i= 1, 2, 3, 4) is a parameter describing the order of the system (3.2); dqdtq= Dq, q =a,b,(i= 1, 2,3, 4) are the sleeping time in the Caputo sense, and x= ( x1(t), x2(t), x3 (t), x 4 (t))T, y= ( y1(t), y2(t), y 3 (t), y4(t))T are the status vectors of system (3.1) and system (3.2) respectively. controller. u(t, x, y) = (u1 (t),u2 (t),u3 (t ),u4 (t)) is the controller. If ai= 0.98, (i= 1, 2, 3, 4) , a= 35, b= 3, c= 12, h= 7,r= 0.5 , then the diagram of the attractors of system (3.1) can be seen in Figure 5.
Figure 5.Diagram of the attractors indicating memory process
Neurotransmitters signals Transportation
Neurotransmitters are chemical agents secreted at the end of axons of nerve cells that diffuse across the synaptic gap and transmit information to adjoining cells such as neurons, muscle cells, and glands, by altering their electrical state or activity. There are many neurotransmitters with a variety of structures and functions.
In order to identify the neuronal functions in sleep and memory consolidation process shown above, the sleeping time step is taken as h = 0.0025s , and T = 30s ,ai = 0.98, (i = 1, 2, 3, 4) , bi = 0.96, (i = 5,L, 9) , a = 35, b = 3, c = 12, h = 7,r = 0.5 , The time taken for the simulation, and the order taking system, to select the system parameters, with the initial state value of system (3.1) is x (0) = (2, 0,1,1) , the initial state value of system (3.2) is y (0) = (-4, -2,1, -5) . Therefore, the sleeping process between system (3.1) and system (3.2) is shown in the following Figure 6:
Figure 6.Modifiable structures error e1 , e2 , e3 , e4 curve between system (3.1) and system (3.2)
According to the definition of neurotransmitters signals transportation, suppose that the neurotransmitters signals transportation is e = x + y . If for any x (0), y (0) satisfy the condition
lim e t¥ = lim t¥ x (t ) + y (t ) = 0 , then we say that system (3.1) and system (3.2) achieve modifiable structures.
On the basis of adaptive control methods, we can give the virtual neurotransmitter by serotonin and acetylcholine systems in central nervous system involving sleep and memory:
| u1 (t) = Dt x1-Dt x1-( a( y2 - y1) + y4 ) + a(x2 - x1 ) + x4 - k1 e1
| u2 (t) = Dt x2 - Dt x2 -( h( y1 - y1y3 + cy2) + hx1-x1x2+cx2-k2e2
| u3 (t) = Dt x2 - Dt x2 -(y y1 2 - by 3)+ x1x2-bx5-k3e3
| u4 (t) = Dt x2 - Dt x2 -(y2 y1 2 - ry 3)+ x2x3-rx4-k4e4
Where e1 = x1 + y1, e2 = x2 + y2 , e3 = x3 + y3 , e4 = x4 + y4 , ki> 0, (i= 1, 2, 3, 4) . If t ¥ ,
Therefore, we achieve the neuronal functions in sleep and memory consolidation process by a number of adaptive robust set of fractional differential equations anti-synchronization indicating memory process.
Chemical and Physical Properties
In this part, mainly through the mathematical method, the preliminary screening of the data in Table 1 for the chemical and physicael propertis of neuron, and respectively by MATLAB and SPSS 22 software to the data provided by principal component analysis, that can only find that the influence factors on the quality of sleep and memory process standard, but can't find out what factors. Then we use the binary logistic regression model to process the data.
First, we extract the data from the above Table 2 to make the 12*7 Table, and then use the principal component analysis method in MATLAB software (The code is shown in the last Annex 1 of this article). We find out that the most influential factors are Formal Charge, Heavy Atom Count, Defined Atom Stereocenter Count. Finally, we analyze the data in Table 2, draw the broken line diagram (The code is shown in the last Annex 2 of this article),which is the following Figure 7, and we find out that the fifth neurotransmitters GABA is the most prominent one19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62.
Conclusions and Future Directions
SWS and REM sleep complement each other to optimize memory integration. In the SWS process in which slow oscillations induce a broad synchronization of neuronal activity, active system consolidation combines newly encoded memory with pre-existing long-term memory to induce conformational changes in their respective expressions. System integration (priority affects explicit coding of behavior-related information) synergizes with global synaptic downscaling, primarily to rule out saturation of synaptic networks. Subsequent REM sleep, characterized by desynchronization of the neural network, may reflect the detachment of the memory system—possibly stabilizing the transformed memory by consolidating undisturbed synapses.
Sleep deprivation suppressed activation of the kinase complex mTORC1 which leads to a decrease in phosphorylation of 4EBP2, leading to disruption of protein translation, reduced neuroplasticity, and ultimately cognitive impairment. Restoring protein synthesis by increasing the amount of phosphorylated 4EBP2 protein in the hippocampus, a function normally performed by mTORC1 can compensate the memory impairment caused by sleep deprivation. Also some alternative mechanisms may actuate as well in the protein synthesis reduction which needs a further studying.
The development of neurotransmitters and its complex functions during sleep and memory process are influenced by numerous factors. In this study, some mathematical speculations have been proposed on the basis of structural and functional characteristics of virtual neuron (especially the physiological phenomena of human beings) with a molecular docking and biomathematical approach to formulate some speculations to the consolidation of sleep and memory process. This could pave a way to formulate more mathematical speculations related with neuron, and finally these data and approaches will be useful for constructing virtual neuron with the help of biomathematics.
Acknowledgments
We would like to express my gratitude to all those who helped us during the writing of this article.
References
- 1.J D SWEATT, HAWKINS K.The molecular neurobiology of the sleep-deprived. , fuzzy brain [J] . Science Signaling 2016, 425-1.
- 2.J C TUDOR, E J DAVIS, PEIXOTO L. (2016) Sleep deprivation impairs memory by attenuating mTORC1-dependent protein synthesis [J]. Science Signaling. 9, 425-1.
- 3.WAGNER U, GAIS S, BORN J.Emotional memory formation is enhanced across sleep intervals with high amounts of rapid eye movement sleep[J]. , Learning & Memory 8(2), 112.
- 4.NISHIDA M, PEARSALL J, R L BUCKNE. (2008) REM sleep, prefrontal theta, and the consolidation of human emotional memory [J]. Cerebral Cortex. 19(5), 1158-1166.
- 5.SMITH C. (2003) The REM sleep window and memory processing in Sleep and brain plasticity [M]. New York:.
- 7.RASCH B, BUCHEL C, GAIS S. (2007) Odor cues during slow-wave sleep prompt declarative memory consolidation. 315-5817.
- 8.GAIS S, PLIHAL W, WAGNER U. (2000) Early sleep triggers memory for early visual discrimination skills [J]. Nature Neuroscience. 3, 12-1335.
- 9.GIUDITTA A, M V A, MONTAGNESE P. (1995) The sequential hypothesis of the function of sleep [J]. Behavioural Brain Research. 69, 1-2.
- 10.P W FRANKLAND, BONTEMPI B. (2005) The organization of recent and remote memories [J]. Nature Reviews Neuroscience. 6(2), 119-130.
- 11.TONONI G, CIRELLI C. (2006) Sleep function and synaptic homeostasis [J]. Sleep Medicine Reviews. 10(1), 49-62.
- 12.MARSHALL L, BORN J. (2007) The contribution of sleep to hippocampus-dependent memory consolidation [J]. Trends in Cognitive Science. 11(10), 442-450.
- 13.V, CIRELLI C, PFISTERGENSKOW M. (2008) Molecular and electrophysiological evidence for net synaptic potentiation in wake and depression in sleep [J]. Nature Neuroscience. 11(2), 200-208.
- 14.M B DASH, C L DOUGLAS, V. (2009) Long-term homeostasis of extracellular glutamate in the rat cerebral cortex across sleep and waking states [J]. , Journal of Neuroscience 29(3), 620-629.
- 15.MARION K, ELIAS W, JONATHAN G.Sleep recalibrates homeostatic and associative synaptic plasticity in the human cortex [J]. , Nature Communication 2016, 12455.
- 16.C G VE, PEIXOTO L, J H CHOI. (2012) Genomic analysis of sleep deprivation reveals translational regulation in the hippocampus [J]. Physiological Genomics. 44-20.
- 19.E J N, S E HYMAN, R J MALENKA. (2001) Molecular Neuropharmacology: Foundation for Clinical Neuroscience[M]. , New York:
- 20.PESTANA M, JARDIM H, CORREIA F. (2001) Renal dopaminergic mechanisms in renal parenchymal diseases and hypertension [J]. Nephrol Dial Transplant. 1(1), 53-59.
- 21.A M M, J W GERRARD, D J BUCHAN. (1960) Glutamic acid derivatives in adult celiac disease. II. Urinary total glutamic acid excretion [J]. Canadian Medical Association Journal. 83, 83-1324.
- 22.BELANGER R, CHANDRAMOHAN N, MISBIN R. (1972) Tyrosine and glutamic acid in plasma and urine of patients with altered thyroid function. 21(9), 855-865.
- 23.RAGGINER C, LECHNER A, BERNECKER C.Reduced urinary glutamate levels are associated with the frequency of migraine attacks in females [J]. , European Journal Neurology 19(8), 1146-1150.
- 24.FIELD T, DIEGO M, HERNANDEZREIF M. (2010) Comorbid depression and anxiety effects on pregnancy and neonatal outcome [J]. Infant Behavior Development. 33(1), 23-29.
- 25.GHADDAR A, K H OMAR, DOKMAK M.Work-related stress and urinary catecholamines among laboratory technicians [J]. , Journal of Occupational Health 55(5), 398-404.
- 26.LIU L, Q L I, N L I. (2011) Simultaneous determination of catecholamines and their metabolites related to Alzheimer’s disease in human urine [J]. , Journal Separation Science 34(10), 1198-1204.
- 27.N J PAI, L, J A BLUMENTHAL. (2015) Association of depressive and anxiety symptoms with 24-hour urinary catecholamines in individuals with untreated high blood pressure [J]. Psychosomatic Medicine. 77(2), 136-144.
- 28.J W HUGHES, WATKINS L, J A BLUMENTHAL. (2004) Depression and anxiety symptoms are related to increased 24-hour urinary norepinephrine excretion among healthy middle-aged women [J]. , Journal of Psychosomatic Research 57(4), 353-358.
- 29.S H KO, J W MAAS, C L BOWDEN. (1983) CSF and urinary biogenic amines and metabolites in depression and mania. A controlled, univariate analysis [J]. Archives General Psychiatry. 40(9), 999-1010.
- 30.C S NI, G D, G P SUTTON. (2001) . Urine GABA levels in ovarian cancer patients: elevated GABA in malignancy 162(1), 27-30.
- 31.M I NICHKOVA, HUISMAN H, P M WYNVEEN.Evaluation of a novel ELISA for serotonin: urinary serotonin as a potential biomarker for depression [J]. , Analytical & Bioanalytical Chemistry 402(4), 1593-600.
- 32.ARUOMA T, BAHORUN L, JEN S. (2003) Neuroprotection by bioactive components in medicinal and food plant extracts [J]. Mutation Research. 544, 2-3.
- 33.ZHAO B, DIRAVIYAM T, X Y ZHANG. (2015) A bio-mathematical approach: Speculations to construct virtual placenta [J]. Applied Mathematics and Computation. 256-344.
- 34.CUMPSON P, SANO NAOKO. (2013) Stability of reference masses V: UV/ozone treatment of gold and platinum. 50(1), 27-36.
- 35.GANESH S, J.MERCY A A. Effects of Phytochemicals extracted from Acanthus Ilicifolius against Staphylococcus aureus: An In-silico approach [J]. , American Journal of Drug Discovery and Development 3(4), 293-297.
- 36.WANG T, X Y ZHANG, Ma XIE W Y Cistanche deserticola Y C.Desert Ginseng”: A Review [J]. , The American Journal of Chinese Medicine 40(6), 1123-1141.
- 37.MERAJ K, M K, N B CHRISTI. (2012) Molecular modeling, docking and ADMET studies towards development of novel Disopyramide analogs for potential inhibition of human voltage gated sodium channel proteins. 8, 23-1139.
- 38.J E WILLIAM. (2007) Computational Models for ADME [J]. Annual Reports in Medicinal Chemistry. 42-449.
- 39.POLLARD H, J C SCHWARTZ. (1987) Histamine neuronal pathways and their functions [J]. Trends in Neurosciences. 10(2), 86-89.
- 40.J T SCHMIDT. (1979) The laminar organization of optic nerve fibres in the tectum of goldfish [J]. , Proceedings of the Royal Society of London 205-1159.
- 41.J T SCHMIDT, J A FREEMAN. (1980) Electrophysiologic evidence that retinotectal synaptic transmission in the goldfish is nicotinic cholinergic [J]. Brain Research. 187(1), 129-142.
- 43.ASANO T, M U I, OGASAWARA N. (1985) Prevention of the Agonist Bindingto y-Aminobutyric Acid B Receptors by Guanine Nucleotides and Islet-activating Protein, Pertussis Toxin, in BovineCerebral Cortex [J]. , Journal of Biological Chemistry 260-23.
- 44.D T Monaghan, C W. (1986) Identification and properties of N-methyl-D-aspartate receptors in rat brain synaptic plasma membranes [J]. Proceedings of the National Academy of Sciences of the United States of America 7533-7536.
- 46.OKOH D, OWOLABI O, EKECHUKWU C. (2016) A regional GNSS-VTEC model over Nigeria using neural networks: A novel approach [J]. Geodesy and Geodynamics. , 7(1) :
- 47.SHEN J, TAN C, ZHANG Y.Discovery of Potent Ligands for Estrogen Receptor β by Structure-Based Virtual Screening [J]. , Journal of Medicinal Chemistry 53(14), 5361-5365.
- 48.X L LI, J. (2013) Stability and Bifurcation Analysis in a System of Four Coupled Neurons with Multiple Delays[J]. Acta Mathematicae Applicatae Sinica (English Series). 29(2), 425-448.
- 49.CHEN S, X J ZHANG, L X. (2015) Histone deacetylase 6 delays motor neuron degeneration by ameliorating the autophagic flux defect in a transgenic mouse model of amyotrophic lateral sclerosis [J]. Neuroscience Bulletin. 31-4.
- 50.URBANSKA M, BLAZEJCZYK M, JAWORSKI J. (2008) Molecular basis of dendritic arborization [J]. Acta neurobiologiae experimentalis. 68(2), 264-288.
- 51.YOUSEFI F, AMOOZANDEH Z.Statistical mechanics and artificial intelligence to model the thermodynamic properties of pure and mixture of ionic liquids[J]. , Chinese Journal of Chemical Engineering 24(12), 1761-1771.
- 52.YOUSEFI F, AMOOZANDEH Z.A new model to predict the densities of nanofluids using statistical mechanics and artificial intelligent plus principal component analysis[J]. , Chinese Journal of Chemical Engineering 25(9), 1273-1281.
- 53.XIE Y, Y M KANG. (2014) Firing properties and synchronization rate in fractional-order Hindmarsh-Rose model neurons [J]. Science China (Technological Sciences). 57(5), 914-922.
- 54.J X ZHAO, H Z XU, Y X TIAN.Effect of electroacupuncture on brain-derived neurotrophic factor mRNA expression in mouse hippocampus following cerebral ischemia-reperfusion injury [J]. , Journal of Traditional Chinese Medicine 33(2), 253-257.
- 55.H Y CUI, FENG C, Y J LIU. (2013) Analysis of prediction performance in wavelet minimum complexity echo state network [J]. The Journal of China Universities of Posts and Telecommunications. 20-4.
- 56.J L BARKER, R M MCBU. (1979) Phenobarbitone modulation of postsynaptic GABA receptor function on cultured mammalian neurons [J]. , Proceedings of the Royal Society of London 319-327.
- 57.B E KOSOFSKY, M E, J H MORRISON.The serotonin and norepinephrine innervation of primary visual cortex in the cynomolgus monkey (Macaca fascicularis) [J]. , Journal of Comparative Neurology 230(2), 168-178.
- 58.V S RAMACHANDRAN, CRONINGOLOMB A, J. (1986) Perception of apparent motion by commissurotomy patients. 320-6060.
- 59.J R BROWN, G W ARBUTHNOTT. (1983) The electrophysiology of dopamine (D2) receptors: a study of the actions of dopamine on corticostriatal transmission. 349-355.
- 60.R Y MOO, F E BLOOM. (1979) Central catecholamine neuron systems: anatomy and physiology of the norepinephrine and epinephrine systems [J]. Annual Review of Neuroscience. 2-113.



