Vibrational, Ultra Violet, Natural Bond Orbital Analysis of E-1 using Quantum Mechanical Computations and Experimental Spectra.
Abstract
The FT-IR, FT-Raman and UV-Vis spectra of E-[1-(3'-methylthienyl)-5-Phenyl-2,4-Pentadiene-3-one (MPPO) were recorded. The optimized molecular bond parameters, harmonic frequencies were calculated using B3LYP method with 6-311++G (d,p) basis set.The various normal modes were precisely assigned with thehelp ofTED calculation. The theoretical spectrograms for FT-IR, FT-Raman and Ultra Violet visible. Spectra of the title molecule had been constructed. The ICT was calculated by means of Natural Bond Orbital analysis. The Non Linear Optical properties related to polarizability and hyperpolarizability based on the finite-field approach were calculated.The band gap energy was calculated using HOMO-LUMO analysis. Furthermore, the Molecular Electrostatic Potential, Mulliken atomic charges and thermodynamic properties of MPPO were also calculated.
Author Contributions
Academic Editor: Dr. Praveen Kumar Sharma, Lovely Professional University, Phagwara, Punjab, India-144411
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2016 Sumathi D., et al
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Chalcones belong to the flavonoid families which are synthesized in factories to preserve the health of plants against infections and parasites. They have attractedincreasing attention due to numerous pharmacological applications 1, 2, 3, 4, 5, 6, 7, 8.It possess various multipronged activities such as anti-microbial 9, anti-depressants 10, anti-plasmodial 11, anti-aids 12, insect anti-feedant activities 13, 14, biological treatment due to its good anti-malaria 15, and in vitro anti-tumor activity 16. It exhibits radical quenching and hydroxyl adducts formation 17. Whilst chalcones are active against various protein targets, modification of the privileged core could lead to novel compounds with specifically targeted inhibitory activity 18.
Chidan Kumar et al., 19 synthesized the high quality single crystal of efficient novel NLO chalcone derivative (2E)-1-(5-chlorothiophen-2-yl)-3-(2,3,4-trimethoxyphenyl) prop-2-en-1-one and its structure was characterized by FTIR, FT-Raman and single crystal XRD techniques. The vibrational wavenumbers were computed using DFT and were assigned on the basis of PED analysis. The geometrical parameters obtained from XRD studywere compared with the calculated values by applying DFT/6-31G (d,p) basis set. Stability of the molecule, hyper conjugative interactions, charge delocalization and intra-molecular hydrogen bond had been studied using NBO analysis. Karunakaran et al., 20 reported the FTIR and FT-Raman spectra of trans-3-(o-hydroxyphenyl-1-phenyl)-2-propen-1-one (or simply 2-hydroxychalcone) were recorded in the regions 4000–400 cm-1 and 3500–100 cm-1, respectively in the solid phase. The vibrational wavenumbers were calculated by HF and DFT/B3LYP methods with 6-311++G (d, p) basis set, using Gaussian 09W program package. A detailed interpretation of the IR and Raman spectra of 2-hydroxychalcone was reported.
In light of the above literature survey, it was clear that inspite of many important applications of MPPO not many works were carried out on this molecule; particularly the complete vibrational analysis supported by quantum computations was not carried out. Hence in this study, FTIR, FT-Raman and UV-Vis. spectral analysis of the compound MPPO was carried out. The NBO analysis, with emphasis on HOMO-LUMO, NLO, MEP, Mulliken’s charges, and various thermodynamic parameters were also calculated using quantum computations by DFT/B3LYP method.
Experimental Details
Synthesis Procedure
Mono benzal acetone (1.46g, 0.01mol) was dissolved in 15 mL of ethanol with slight warning. To this hot solution methyl thiophen-2-carboxaldehyde (1.4 mL, 0.01mol) and few drops of solution hydroxide solution 10%) were added. The solution gradually turned red on warming and yellow crystals separated. The product was filtered off and recrystalised twice from ethanol. The yield and melting point of the crystal is 76% and 148 respectively.
Instrumentation
FT-IR, FT-Raman and UV–Vis Spectra
The FT-IR spectrum on an IFS 66v spectrophotometer of MPPO was recorded in the spectral region between 400–4000 cm-1 using the KBr pellet technique. The spectrum was recorded at room temperature with a scanning speed of 10 cm-1 per minute and at the spectral resolution of 2.0 cm-1 in the Department of Chemistry, Jamal Mohamed College, Trichy, Tamilnadu, India. The FT-Raman spectrum of title compound was recorded using the 1064 nm line of an Nd: YAG laser as excitation wavelength in the region 50–3500 cm-1 on Bruker model IFS 66V spectrophotometer equipped with an FRA 106 FT-Raman module accessory and at spectral resolution of 4 cm-1. The FT-Raman spectral measurement was carried out from SAIF Laboratory, IIT Madras, Tamilnadu, India. The UV-Vis absorption spectrum of MPPO was recorded in the range of 200–500 nm using a Shimadzu – 2600 spectrometer in the Department of Chemistry, Jamal Mohamed College, Trichy-20. The UV pattern was taken from a 10-5 molar solution of MPPO dissolved in benzene.
Computational Details
For meeting the requirements of both accuracy and computing economy, theoretical methods and basis sets should be considered. DFT had proved to be extremely useful in treating electronic structure of molecules. The density functional theory parameter hybrid model DFT/B3LYP/6 basis set was adopted to calculate the properties of the title molecule in this work. 50All the calculations were performed using the Gaussian 03W program package 21 with the default convergence criteria without any constraint on the geometry 22. It should be noted that Gaussian 03W package did not calculate the Raman intensities. The Raman activities were transformed into Raman intensities using Raint program 23.
Results and Discussion
Molecular Geometry
The optimized geometrical parameters for MPPO are calculated using B3LYP/6-311++G(d,p) level of calculation and are presented in Table 1 in accordance with atom numbering given in Figure 1. The title molecule consists of thiophene and phenyl ring fused by carbonyl group. In MPPO the C=O group plays an important role. The carbonyl (C16=O17) bond length is observed at 1.190 Å24, whereas the calculated bond length is about 1.235Å.In thiophen ring, the two C-S bond lengths are found at 1.707 and 1.728Å.In this study, the C1-S5(1.762Å) and C4-S5(1.717Å) bond lengths are differ by 0.045Å and the bond angles C2-C1-S5(110.34˚) and C3-C4-S5(112.86˚) also differ by 2.52˚,which is due to the shortening of bond distance between atoms C1 and S5 atoms. The bond angle O17-C16-C18(124.37˚) is positively (~2.98˚) from C14-C16-O17(121.39˚). This may be due the intra- or inter-molecular interaction S---O---H. From the theoretical values, it is found that most of the optimized bond parameters are slightly greater than the experimental values, since the calculation has been done on a single molecule in gaseous state.Although the differences, calculated geometrical parameters represent a good approximation and they can be used as foundation to calculate the other parameters, such as vibrational frequencies and thermodynamics properties.The calculated bond parameters are compared with experimental values24, 25. The optimized structure is planar as it is evident from the dihedral angles C12C14C16O17=0.00° & C12C14C16C18=-180.00°; C14C16C18C20=180.00° & O17C16C18C20=-0.00°;C2C1C12C14=179.99˚& S5C1C12C14=-0.00˚ and C18C20C22C23=-179. 99˚ & C18C20C22C24=0.00˚ are listed in Table 1.
Figure 1.The optimized molecular structure of MPPO
| Parameters | B3LYP/ 6-311++G(d,p) | XRD a,b,c,d |
| Bond Lengths (Å) | ||
| C1-C2 | 1.401 | 1.375a, 1.362b |
| C1-S5 | 1.762 | 1.728b |
| C1-C12 | 1.436 | |
| C2-C3 | 1.415 | 1.431a, 1.392b |
| C2-C8 | 1.507 | |
| C3-C4 | 1.372 | 1.37d, 1.345b |
| C3-H6 | 1.083 | 1.081c |
| C4-S5 | 1.717 | 1.717a, 1.707b, 1.714d |
| C4-C7 | 1.081 | |
| C8-H9 | 1.091 | |
| C8-H10 | 1.094 | |
| C8-H11 | 1.094 | |
| C12-H13 | 1.087 | 0.950 (6)c |
| C12-C14 | 1.361 | |
| C14-H15 | 1.085 | 0.951 (8)c |
| C14-C16 | 1.467 | |
| C16-C18 | 1.479 | |
| C16-O17 | 1.235 | 1.190 (3)c |
| C18-H19 | 1.086 | 0.951 (8)c |
| C18-C20 | 1.358 | |
| C20-H21 | 1.089 | 0.950 (6)c |
| C20-C22 | 1.463 | |
| C22-C23 | 1.412 | 1.366 (4)c |
| C22-C24 | 1.409 | |
| C23-C25 | 1.389 | |
| C23-H26 | 1.085 | 0.954 (2)c |
| C24-C27 | 1.391 | |
| C24-H28 | 1.08 | 0.949 (2)c |
| C25-C29 | 1.395 | |
| C25-H30 | 1.084 | 0.950 (3)c |
| C27-C29 | 1.395 | |
| C27-H31 | 1.084 | 0.950 (2)c |
| C29-H32 | 1.085 | 0.950 (3)c |
| Bond Angles (°) | ||
| C2-C1-S5 | 110.34 | 111.80a |
| C2-C1-C12 | 122.15 | |
| S5-C1-C12 | 127.51 | |
| C1-C2-C3 | 112.64 | 112.20a, 114.72b, 112.5d |
| C1-C2-C8 | 124.93 | |
| C3-C2-C8 | 122.43 | |
| C2-C3-C4 | 112.9 | 112.05b |
| C2-C3-H6 | 123.42 | 123.3d |
| C4-C3-H6 | 123.68 | |
| C3-C4-S5 | 112.86 | 112.13b |
| C3-C4-H7 | 127.42 | |
| S5-C4-H7 | 119.72 | |
| C1-S5-C4 | 91.27 | 92.00a, 91.81b, 92.2d |
| C2-C8-H9 | 110.2 | |
| C2-C8-H10 | 111.96 | |
| C2-C8-H11 | 111.96 | |
| H9-C8-H10 | 107.48 | |
| H9-C8-H11 | 107.48 | |
| H10-C8-H11 | 107.55 | |
| C1-C12-H13 | 110.92 | |
| C1-C12-C14 | 135.87 | |
| H13-C12-C14 | 113.2 | 121.2 (6)c |
| C12-C14-H15 | 115.61 | 116.1 (7)c |
| C12-C14-C16 | 128.31 | 127.7 (6)c |
| H15-C14-C16 | 116.08 | 116.3 (6)c |
| C14-C16-O17 | 121.39 | 106.5 (3)c |
| C14-C16-C18 | 114.24 | 147.1 (4)c |
| O17-C16-C18 | 124.37 | 106.5 (3)c |
| C16-C18-H19 | 113.14 | 116.3 (6)c |
| C16-C18-C20 | 132.74 | 127.7 (6)c |
| H19-C18-C20 | 114.12 | 116.1 (7)c |
| C18-C20-H21 | 112.56 | 121.2 (6)c |
| C18-C20-C22 | 136.42 | 117.6 (5)c |
| H21-C20-C22 | 111.03 | 121.2 (4)c |
| C20-C22-C23 | 116.08 | 140.3 (3)c |
| C20-C22-C24 | 125.99 | 101.8 (3)c |
| C23-C22-C24 | 117.93 | 117.5 (2)c |
| C22-C23-C25 | 121.5 | 121.2 (3)c |
| C22-C23-H26 | 119.09 | 119.4 (3)c |
| C25-C23-H26 | 119.41 | 119.4 (3)c |
| C22-C24-C27 | 120.34 | 120.4 (2)c |
| C22-C24-H28 | 118.84 | 119.8 (2)c |
| C27-C24-H28 | 120.82 | 119.7 (2)c |
| C23-C25-C29 | 119.76 | 120.4 (3)c |
| C23-C25-H30 | 119.96 | 119.8 (3)c |
| C29-C25-H30 | 120.29 | 119.8 (3)c |
| C24-C27-C29 | 120.88 | 120.8 (2)c |
| C24-C27-H31 | 119.27 | 119.6 (3)c |
| C29-C27-H31 | 119.85 | 119.6 (3)c |
| C25-C29-C27 | 119.59 | 119.7 (3)c |
| C25-C29-H32 | 120.15 | 120.2 (3)c |
| C27-C29-H32 | 120.26 | 120.2 (3)c |
| Dihedral Angles (°) | ||
| S5-C1-C2-C3 | 0 | |
| S5-C1-C2-C8 | 180 | |
| C12-C1-C2-C3 | 180 | |
| C12-C1-C2-C8 | 0 | |
| C2-C1-S5-C4 | 0 | |
| C12-C1-S5-C4 | -180 | |
| C2-C1-C12-H13 | 0 | |
| C2-C1-C12-C14 | 179.99 | |
| S5-C1-C12-H13 | 179.99 | |
| S5-C1-C12-C14 | 0 | |
| C1-C2-C3-C4 | 0 | |
| C1-C2-C3-H6 | 180 | |
| C8-C2-C3-C4 | -179.99 | |
| C8-C2-C3-H6 | 0 | |
| C1-C2-C8-H9 | -180 | |
| C1-C2-C8-H10 | 60.43 | |
| C1-C2-C8-H11 | -60.44 | |
| C3-C2-C8-H9 | 0 | |
| C3-C2-C8-H10 | -119.57 | |
| C3-C2-C8-H11 | 119.56 | |
| C2-C3-C4-S5 | 0 | |
| C2-C3-C4-O7 | -180 | |
| H6-C3-C4-S5 | -180 | |
| H6-C3-C4-H7 | 0 | |
| C3-C4-S5-C1 | 0 | |
| H7-C4-S5-C1 | 180 | |
| C1-C12-C14-H15 | 179.99 | |
| C1-C12-C14-C16 | 0 | |
| H13-C12-C14-H15 | 0 | |
| H13-C12-C14-C16 | 179.99 | |
| C12-C14-C16-O17 | 0 | |
| C12-C14-C16-C18 | -180 | |
| H15-C14-C16-O17 | 180 | |
| H15-C14-C16-C18 | 0 | |
| C14-C16-C18-H19 | 0 | |
| C14-C16-C18-C20 | 180 | |
| O17-C16-C18-H19 | 179.99 | |
| O17-C16-C18-C20 | 0 | |
| C16-C18-C20-H21 | 180 | |
| C16-C18-C20-C22 | 0 | |
| H19-C18-C20-H21 | 0 | |
| H19-C18-C20-C22 | 179.99 | |
| C18-C20-C22-C23 | -179.99 | |
| C18-C20-C22-C24 | 0 | |
| H21-C20-C22-C23 | 0 | |
| H21-C20-C22-C24 | -179.99 | |
| C20-C22-C23-C25 | 180 | |
| C20-C22-C23-H26 | 0 | |
| C24-C22-C23-C25 | 0 | |
| C24-C22-C23-H26 | 180 | |
| C20-C22-C24-C27 | -180 | |
| C20-C22-C24-H28 | 0 | |
| C23-C22-C24-C27 | 0 | |
| C23-C22-C24-H28 | -180 | |
| C22-C23-C25-C29 | 0 | |
| C22-C23-C25-H30 | -180 | |
| H26-C23-C25-C29 | 180 | |
| H26-C23-C25-H30 | 0 | |
| C22-C24-C27-C29 | 0 | |
| C22-C24-C27-H31 | 180 | |
| H28-C24-C27-C29 | 180 | |
| H28-C24-C27-H31 | 0 | |
| C23-C25-C29-C27 | 0 | |
| C23-C25-C29-H32 | -180 | |
| H30-C25-C29-C27 | 180 | |
| H30-C25-C29-H32 | 0 | |
| C24-C27-C29-C25 | 0 | |
| C24-C27-C29-H32 | 180 | |
| H31-C27-C29-C25 | 180 | |
| H31-C27-C29-H32 | 0 |
Vibrational Assignments
The molecule MPPO belongs to C1 point group symmetry. This molecule has 32 atoms and hence90 normal modes of fundamental vibrations are possible, which span the irreduciable representation:61A'+29A''. All the 90 fundamental modes are active in both IR and Raman. The vibrational analysis of MPPO is performed on the basis of the characteristic vibrations of carbonyl, methyl, thiophene, pentadiene and phenyl ring modes. In this study, the harmonic vibrational frequencies are calculated at B3LYP level using 6-311++G(d,p)basis set have been collected in Table 2. Some discrepancies could be identified in between harmonic and experimental frequencies, which are scaled down by proper scale factor 26. To find the exact vibrational behavior of this molecule the TED analysis was performed on the gas phase. The combined experimental and theoretical spectra are shown in Figure 2,Figure 3.
Table 2. The experimental and calculated frequencies of MPPO using B3LYP/6-311++G(d,p) level of basis set harmonic frequencies (cm−1) IR Raman intensities (Km/mol) reduced masses (amu) and force constants (mdynA°−1)| Mode No | Calculated | Observed Frequencies (cm-1) | IR Intensity | Raman Intensity | Reduced Masses | Force Consts | Vibrational Assignments≥10% (TED)d | ||
| Frequencies (cm-1) | |||||||||
| Un Scaled | Scaleda | FT-IR | FT-Raman | Rel.b | Rel.c | ||||
| 1 | 3231 | 3104 | 0.03 | 0.61 | 1.1 | 6.75 | νC24H28(95) | ||
| 2 | 3225 | 3098 | 12.04 | 0.37 | 1.09 | 6.7 | νC14H15(98) | ||
| 3 | 3193 | 3068 | 2.69 | 0.6 | 1.09 | 6.55 | νC8H11(94) | ||
| 4 | 3188 | 3063 | 3064 vw | 3059 vw | 4.7 | 1.04 | 1.1 | 6.57 | νC8H9(83) |
| 5 | 3177 | 3052 | 4.94 | 0.33 | 1.09 | 6.5 | νC8H10(85) | ||
| 6 | 3172 | 3047 | 4.12 | 0.59 | 1.09 | 6.46 | νC18H19(87) | ||
| 7 | 3165 | 3041 | 0.42 | 0.38 | 1.09 | 6.42 | νC23H26(84) | ||
| 8 | 3158 | 3034 | 0.99 | 0.13 | 1.09 | 6.38 | νC12H13(72) | ||
| 9 | 3151 | 3027 | 3021 vw | 0.77 | 0.08 | 1.09 | 6.37 | νC3H6(75) | |
| 10 | 3139 | 3016 | 0.87 | 0.15 | 1.08 | 6.29 | νC20H21(83) | ||
| 11 | 3111 | 2989 | 1.45 | 0.17 | 1.08 | 6.19 | νC4H7(85) | ||
| 12 | 3106 | 2984 | 3.59 | 0.28 | 1.1 | 6.25 | νC25H30(92) | ||
| 13 | 3073 | 2953 | 2919 w | 2921 vw | 2.57 | 0.27 | 1.1 | 6.13 | νC27H31(100) |
| 14 | 3025 | 2907 | 2843 vw | 6.13 | 1.12 | 1.04 | 5.6 | νC29H32(92) | |
| 15 | 1696 | 1629 | 1647 s | 1647 vw | 12.94 | 3.2 | 7.03 | 11.91 | νC16O17(85) |
| 16 | 1642 | 1578 | 1617 m | 1616 w | 17.67 | 1.55 | 5.53 | 8.78 | νC24C27(18)+νC23C25(25)+νC25C29(14)+βH26C23C25(15) |
| 17 | 1624 | 1560 | 1586 vs | 1584 vs | 100 | 21.83 | 5.42 | 8.43 | νC4C3(32)+νC24C27(12) |
| 18 | 1604 | 1541 | 1550 m | 1551 vw | 98.37 | 3.24 | 5.33 | 8.08 | νC18C20(46)+βH32C29C27(20) |
| 19 | 1583 | 1521 | 4.3 | 100 | 7.4 | 10.93 | νC16O17(66) | ||
| 20 | 1534 | 1474 | 1492 m | 1494 vw | 5.18 | 0.67 | 3.93 | 5.44 | νC16C18(45) |
| 21 | 1524 | 1464 | 1.92 | 3.21 | 2.16 | 2.95 | νC12C14(12)+βH15C14C16(50) | ||
| 22 | 1504 | 1445 | 1447 w | 2.05 | 1.15 | 1.19 | 1.58 | βH10C8H11(60)+βH13C12C14(10) | |
| 23 | 1495 | 1437 | 5.7 | 7.89 | 2.04 | 2.69 | βH13C12C14(42) | ||
| 24 | 1490 | 1432 | 1.67 | 0.22 | 1.04 | 1.36 | βH9C8H10(78)+τH9C8C2C3(10)+τH10C8C2C3(10) | ||
| 25 | 1486 | 1428 | 2.28 | 3.84 | 1.72 | 2.24 | βH21C20C22(60) | ||
| 26 | 1467 | 1409 | 1404 m | 1406 vw | 2.2 | 0.77 | 1.82 | 2.31 | βH19C18C20(58) |
| 27 | 1426 | 1370 | 8.06 | 2.12 | 2.81 | 3.36 | νC2C3(24)+νC1C2(14)+βH6C3C4(25) | ||
| 28 | 1412 | 1357 | 0.31 | 0.87 | 1.28 | 1.51 | βH9C8H10(88) | ||
| 29 | 1384 | 1330 | 1334 m | 13.7 | 9.15 | 2.76 | 3.12 | νC14C16(50) | |
| 30 | 1381 | 1327 | 5.07 | 8 | 1.45 | 1.63 | βH21C20C22(65) | ||
| 31 | 1360 | 1307 | 1314 w | 0.1 | 4.73 | 2.13 | 2.32 | νC18C20(14)+νC23C25(18)+βH26C23C25(40) | |
| 32 | 1342 | 1289 | 20.27 | 7.27 | 1.38 | 1.46 | βH13C12C14(58) | ||
| 33 | 1299 | 1248 | 1258 w | 0.51 | 0.51 | 1.83 | 1.82 | νC22C24(34)+βH28C24C27(20) | |
| 34 | 1261 | 1212 | 1193 m | 0.44 | 0.02 | 1.76 | 1.65 | νC2C8(15)+βH6C3C4(52) | |
| 35 | 1216 | 1168 | 1183 m | 1185 w | 3.88 | 0.24 | 1.25 | 1.09 | βH26C23C25(55) |
| 36 | 1205 | 1158 | 0.63 | 4.87 | 2.34 | 2 | νC20C22(25) | ||
| 37 | 1195 | 1148 | 4.24 | 0.45 | 2.1 | 1.77 | νC1C2(14)+νC2C3(10)+βH13C12C14(12) | ||
| 38 | 1184 | 1137 | 0.01 | 0.16 | 1.11 | 0.92 | βH32C29C27(60) | ||
| 39 | 1119 | 1075 | 1090 w | 1090 m | 36.76 | 1.15 | 1.92 | 1.42 | νC18C20(12)+νC16C18(24)+βH19C18C20(12)+βH7C4S5(16) |
| 40 | 1110 | 1066 | 0.16 | 0.21 | 1.35 | 0.98 | νC2C3(10)+βH7C4S5(50) | ||
| 41 | 1097 | 1054 | 50.3 | 0.07 | 2.95 | 2.09 | νC16C18(26) | ||
| 42 | 1052 | 1011 | 1012 m | 0.45 | 1.12 | 2.32 | 1.51 | νC25C29(48)+βH26C23C25(18)+βC23C25C29(12) | |
| 43 | 1051 | 1010 | 0.17 | 0.03 | 1.5 | 0.98 | βH9C8H11(22)+τH9C8C2C3(34)+τH10C8C2C3(34) | ||
| 44 | 1039 | 998 | 1000 w | 1.25 | 0.56 | 1.78 | 1.13 | νC2C8(10)+βH9C8H10(14)+τH9C8C2C3(20)+τH10C8C2C3(20) | |
| 45 | 1032 | 991 | 985 vw | 0.27 | 0.01 | 1.31 | 0.82 | τH28C24C27C29(72) | |
| 46 | 1019 | 979 | 0 | 0.16 | 1.28 | 0.79 | τH19C18C20C22(78) | ||
| 47 | 1015 | 976 | 0.42 | 5.13 | 6.19 | 3.76 | νC27C29(22)+βC24C27C29(50) | ||
| 48 | 997 | 958 | 0.01 | 0.24 | 1.29 | 0.75 | τH13C12C14C16(80) | ||
| 49 | 994 | 955 | 0.09 | 0.02 | 1.32 | 0.77 | τH32C29C27C24(70) | ||
| 50 | 973 | 935 | 2.46 | 0.51 | 2.83 | 1.58 | νC12C14(20) | ||
| 51 | 953 | 916 | 1.09 | 0.01 | 1.4 | 0.75 | τH26C23C25C29(78) | ||
| 52 | 930 | 894 | 1.52 | 0.64 | 3.7 | 1.89 | νC12C14(15)+βC14C16C18(22) | ||
| 53 | 897 | 862 | 0.44 | 0.02 | 1.28 | 0.61 | τH6C3C4S5(76) | ||
| 54 | 865 | 831 | 834 vw | 1.65 | 0 | 1.37 | 0.6 | τH19C18C20C22(78) | |
| 55 | 859 | 825 | 8.59 | 0.02 | 2.06 | 0.89 | τH19C18C20C22(22)+τC1C12C14C16(18)+τH13C12C14C16(20)+τH30C25C29C27(14) | ||
| 56 | 844 | 811 | 812 m | 8.45 | 0.33 | 4.61 | 1.93 | νC4S5(42)+βC1C2C3(15) | |
| 57 | 830 | 797 | 2.64 | 0.21 | 5.16 | 2.09 | νC4S5(28)+βC1C2C3(18) | ||
| 58 | 809 | 777 | 760 vw | 2.51 | 0.04 | 1.97 | 0.76 | τH13C12C14C16(12)+τH30C25C29C27(40)+τC22C24C27C29(12) | |
| 59 | 767 | 737 | 0 | 0.07 | 1.65 | 0.57 | τC1C12C14C16(26)+τH13C12C14C16(38) | ||
| 60 | 763 | 733 | 724 vw | 0.55 | 2.08 | 5.71 | 1.96 | νC20C22(18)+βC18C16O17(36) | |
| 61 | 734 | 705 | 13.14 | 0.03 | 1.23 | 0.39 | τH6C3C4S5(75) | ||
| 62 | 718 | 689 | 697 w | 691 vvw | 2.72 | 0.02 | 1.34 | 0.41 | τH19C18C20C22(44)+τC1C12C14C16(14)+τH30C25C29C27(26) |
| 63 | 715 | 687 | 0.12 | 7.15 | 6.89 | 2.07 | νC4S5(32)+βC1C2C3(22)+βC2C3C4(12) | ||
| 64 | 697 | 670 | 3.56 | 0.03 | 2.52 | 0.72 | τH28C24C27C29(12)+τC22C24C27C29(48) | ||
| 65 | 663 | 637 | 634 vvw | 0.04 | 0.55 | 6.25 | 1.62 | νC12C14(22)+βC23C25C29(12)+βC1C2C3(14)+βC2C3C4(10) | |
| 66 | 632 | 607 | 0.21 | 0.43 | 6.31 | 1.48 | βC18C20C22(62) | ||
| 67 | 625 | 601 | 0.14 | 0.11 | 2.93 | 0.68 | τH13C12C14C16(12)+ГC8C1C3C2(58) | ||
| 68 | 603 | 579 | 0.6 | 0.83 | 5.49 | 1.18 | νC1C2(35)+βC2C1C12(20) | ||
| 69 | 549 | 528 | 531 vw | 0.28 | 0.31 | 7.08 | 1.26 | βC1C2C3(10)+βC1C12C14(24) | |
| 70 | 529 | 508 | 0.23 | 1.58 | 6.2 | 1.02 | νC2C8(15)+βC1C2C3(50) | ||
| 71 | 526 | 505 | 0.08 | 0.42 | 3.06 | 0.5 | ГC20C23C24C22(65) | ||
| 72 | 490 | 470 | 455 m | 2.31 | 0.02 | 3.2 | 0.45 | ГC20C23C24C22(60) | |
| 73 | 463 | 445 | 424 w | 6.62 | 0.49 | 6.05 | 0.76 | βC22C24C27(10)+βC1C12C14(30) | |
| 74 | 423 | 407 | 402 vw | 0.3 | 0.24 | 3.28 | 0.35 | τC1C2C3C4(44)+τC23C25C29C27(22) | |
| 75 | 412 | 395 | 0.14 | 0.15 | 3.14 | 0.31 | τC1C2C3C4(20)+τC23C25C29C27(42) | ||
| 76 | 358 | 344 | 0.12 | 0.53 | 3.61 | 0.27 | βC3C2C8(60) | ||
| 77 | 350 | 336 | 0.34 | 0.13 | 3.4 | 0.25 | τC16C18C20C22(55) | ||
| 78 | 268 | 258 | 1.22 | 0.28 | 8.91 | 0.38 | βC14C16C18(60) | ||
| 79 | 237 | 228 | 0.5 | 0.07 | 3.64 | 0.12 | ГC8C1C3C2(62)+ГC20C23C24C22(15) | ||
| 80 | 226 | 217 | 0.31 | 5.93 | 7.6 | 0.23 | βC18C20C22(55) | ||
| 81 | 194 | 187 | 0.25 | 0.45 | 4.55 | 0.1 | νC3C4(12)+βC2C1C12(40) | ||
| 82 | 180 | 173 | 164 vw | 0.19 | 0.43 | 3.83 | 0.07 | τC24C27C29C25(55)+ГC20C23C24C22(12) | |
| 83 | 153 | 147 | 0.21 | 0.14 | 4.72 | 0.07 | ГC8C1C3C2(14)+ГC20C23C24C22(60) | ||
| 84 | 145 | 139 | 0.27 | 0.42 | 5.54 | 0.07 | βC22C24C27(10)+βC24C27C29 (65) | ||
| 85 | 110 | 106 | 0.02 | 0.27 | 1.03 | 0.01 | τH9C8C2C3(95) | ||
| 86 | 78 | 75 | 69 w | 0.71 | 0.1 | 7.08 | 0.03 | ГC20C23C24C22(75) | |
| 87 | 52 | 50 | 0.03 | 5.81 | 6.66 | 0.01 | βC12C14C16(78) | ||
| 88 | 47 | 45 | 0.02 | 3.67 | 4.51 | 0.01 | τC2C1C12C14(72) | ||
| 89 | 28 | 27 | 0.01 | 1.93 | 4.08 | 0 | τC3C2C1C12(62) | ||
| 90 | 14 | 13 | 0.22 | 0.06 | 5.21 | 0 | τC1C12C14C16(80) |
Figure 2.The combined theoretical and experimental FT-IR spectra of MPPO
Figure 3.The combined theoretical and experimental FT-Raman spectra of MPPO
C=O Vibrations
The carbonyl stretching frequency has been most extensively studied by IR spectroscopy 27. The multiply bonded group is highly polar and therefore gives rise to an intense IR absorption band. The carbon–oxygen double bond is formed by Pπ–Pπ bonding between carbon and oxygen atoms. The loan pair of electrons on oxygen also determines the nature the carbonyl group. The νC=O mode of carboxylic acids is identical to the νC=O mode in ketones, which is expected in the region 1660-1740 cm-128.The stretching vibration of carbonyl group C=O can be observed as a very strong band in both FTIR and FT-Raman spectra at 1665cm-129, 30.According to above literature strong band observed in FTIR spectrum at 1647 cm-1 and weak band in FT-Raman spectrum at 1647 cm-1 is assigned to C=O stretching vibration.The value of this band is calculated 1629cm-1 (mode no:15) with a TED of 85%. This assignment is in moderate agreement with literature 31 in the case of thiophene-2-carohydrazide. The observed very weak band in FT-Raman at 724cm-1 is ascribed to βC16=O17 mode, while its correspondingharmonic frequency is 733cm-1 (mode no:60). These assignments are also supported by the literature31in addition to TED (36%) output.
C-S Vibrations
The C-S stretching vibration is well known to mix with neighboring modes 32.Literature survey revealed that ν(C-S) vibration of thiophene ring was observed in the region 719-842cm-133. The harmonic wave numbers 811,687 (mode nos:56,63) and FTIR band observed at 812 cm-1 are assigned to νC-S modes. These modes are mixed with βCCC modes and also have considerable TED (>32%) values. Furthermore, these assignments also find support from the literature34.
Methyl Group Vibrations
Methyl groups are generally referred to as electron donating substituents in the aliphatic and aromatic ring system 35. For the assignments of CH3 group, basically nine fundamentals can be associated with each CH3 group namely: the symmetrical stretching in CH3 (CH3sym. stretching); anti-symmetrical stretching (CH3asym. stretching); the symmetrical (CH3 sym. deformation) and anti-symmetrical (CH3asy. deformation) deformation modes; the in-plane rocking (CH3ipr), out-of-plane rocking (CH3opr) and twisting (CH3) bending modes. The asymmetric/symmetric CH stretching modes of CH3 are expected in the region around 2980/2870 cm-1, respectively 36, 37, 38, 39. The CH3asy/sym frequencies are established at 3068, 3063/3052 cm-1 (mode nos: 3, 4/5), respectively. This assignment is further supported by observed bands (FTIR: 3064/FT-Raman: 3059 cm-1) in addition to TED values (>83%). The asymmetric/symmetric CH3 bending vibrations usually occur in the regions 1430-1470cm-1/1360-1400 cm-1, respectively 40. In accordance with the above literature, the harmonic bands 1445, 1432/1357cm-1 (mode nos: 22, 24/28) are respectively assigned to βasy CH/βsy CH of CH3 group. Further, these assignments are supported from observed FTIR band: 1447 cm-1 in addition to TED values (>60%).
The CH3 rocking modes usually appeared39in the region 1010-1070 cm-1. The bands established at 983 and 966 cm-1 in FT-Raman and FTIR were assigned to CH3 in-plane and out-of-plane rocking modes, respectively 41. The value of these bands are calculated at 1010 and 998 cm-1 (mode nos: 43 and 44) with considerable TED values (68% & 40%). The mode no: 44 coincideswith observed FT-Raman band (1000 cm-1). These assignments find support from the literatureSubramanian et al., 42 and were within the frequency intervals given by Varsanyi et al.,43. The CH3 torsional mode was assigned at 168 cm-142. It should be mentioned here, that this mode is pure with 95% TED value. Hence the mode no: 85 is undoubtedly assigned to τCH3 mode.
C=C, C-C Vibrations
The ring C=C and C-C stretching vibrationsknown as semicircle stretching usually occur in the region 1450–1625 cm-144, 45. Varsanyi et al., 46have assigned the νC-C vibrations in the regions of 1590-1625,1575-1590,1470-1540,1430-1460 and 1280-1380cm-1 with variable intensity of bands. The ring νC-Cmodes occur in the region 1530-1625cm-142.In this region there are two (or) three bands due to skeletal vibrations, the strongest usually being at about 1500cm-1 for aromatic six member rings. Hence, the bands of strong to medium intensity in FTIR at1617 (m),1586 (vs) & 1012 (m) cm-1 and FT-Raman at 1616(w),1584(vs),1314(w) & 1258 (w) cm-1 are assigned to C-C stretching vibrations of benzene moiety. The frequencies assigned to νC-C modes agree well with the literature 44, 45 and also in line with the harmonic wavenumbers in the range 1578-976cm-1(mode nos:16,17,31,33,42, 47) in addition to TED values.
The βCCC and ΓCCCmodes are associated with smaller force constant than the stretching one and hence assigned to lower frequencies. The βCCC modes of benzene ring are expected to appear with considerable intensity under the reduced symmetry.The C-C-C in-plane bending vibrations can be observed in the FTIR spectrum at 1012cm-1 and in FT-Raman spectrum at 634 cm-1.Similarly the C-C-C out-of-plane bending modes can be observed as a medium band in FTIR spectrum at 455cm-1. These results are supported by computed wavenumbers 1011, 976, 637 and 670, 505, 470 cm-1 (mode nos:42, 47, 65 and 64, 71,72), respectively in addition to literature values 39.
Literature survey reveals that the C-C stretching vibrations in substituted thiophen rings were reported in ranges of 1329-1431,1420-1501 and 1419-1519cm-128, 30, 31. Inour case the very strong bands at 1586/1584 cm-1 in FTIR/FT-Raman and harmonic bands at 1560, 1370 and 1148 cm-1( mode nos:17,27 &37) are assigned to νC-C modes in MPPO. These assignments are having considerable TED values(>24%). As it is evident from the Table 2, the βCCC modes: 811,687 cm-1(mode nos:56,63) are mixed with νC-S modes. Mode no: 56 is in good agreement with observed FTIR band at 812cm-1 in addition to TED output (>15%). These assignments are also in accordance with literature28, 30, 31. The mode nos:67,79 (601, 228cm-1) are designated as ΓCCC modes of thiophen moiety with considerable TED values (>58%).
The harmonic frequencies established at 1541,1474,1330,1075 cm-1(mode nos: 18,20,29,39) and 1158cm-1, (mode no:36) are attributed to νCC modes of pentadiene moietyand νC20-C22,νC1-C12 modes, respectively. These assignments are having considerable TED values (>25%) and also find support from the observed bands:1550,1492, 1334,1090 cm-1(FTIR)/1551,1494,1090 cm-1 FT-Raman. The mode no:34 (1212/1193cm-1:FTIR) is attributed νC2-C8 mode. The harmonic wavenumbers:894,607,528, 445 cm-1(mode nos:52,66,69,73) and 689,737,336cm-1(mode nos:62,59,77) are respectively assigned to βCCC and τCCC modes of pentadiene, in which mode nos:69,73 and 62 are agreeable with observed bands: (FT-Raman/FTIR)531/424cm-1 and 691/697cm-1. These assignment are also having considerable TED values.
C-H Vibrations
The νC-H modes of hetero aromatic structure are expected to occur in the range of 3000-3100cm-1 with some weak bands. The vibrational band in this region are not affected appreciably by the nature of the substituent’s 43, 44. In MPPO,the weak bands at 2919 (2921:FT-Raman), 2843cm-1 in FTIR spectrum have been assigned to νC-H vibrations. The present theoretical calculation by B3LYP/6-311++G(d,p) method places this modes at 3104,3041,2984,2953,2907cm-1 (mode nos:1,7,12,13,14). This shows that the bands have appeared at the expected position of the spectra, except the last band (2843cm-1) which is slightly less.The reduction in the frequency value is naturally due to the presence of CH3 group inMPPO.
In aromatic compounds the C-H in-plane /out-of-plane bending frequencies appear in the ranges of 1000-1300/750-1000cm-1, respectively45, 46. In this work the βC-H modes are assigned in the range 1307-1011cm-1 (modenos:31,33,35,38,42), which are in agreement with observed bands in FTIR at 1183,1012 cm-1 and in FT-Raman at 1314,1258,1185 cm-1. The calculated frequencies 991-777cm-1 (mode nos: 45, 49, 51, 55, 58) for C-H out-of-plane bending modes fall in the FTIR values of 985,760cm-1. These assignments are find support from the Varsanyi 46 and also find support from TED values. Hence, among the CH vibrations, only the CH stretching modes are found influenced by the substitutional group, whereas the βCH and ΓCHmodes are remain unaffected.
The C-H stretching modes are expected to appear with multiple weak bands in the frequency range 3000-3100cm-1. These bands are not affected appreciably by the nature of the substituent’s 43, 47.The νC-H modes were observed at 3011,3062 and 3072 cm-1 in FTIR spectrum by Balachandran et al., 31. Based on the above conclusion, the calculated frequencies 3027,2989cm-1 (mode nos: 9, 11) are assigned to νC-H vibrations of thiophen moiety. These assignments are aggreable with observed FTIR band at 3021 cm-1 and also find support from TED values (>75%).The C-H in-plane bending modes appeared by sharp but weak to medium bands in the range 1100-1500cm-1 and the bands were not sensitive to the nature of substitutent’s. The ΓC-H out-of-plane bending modes were expected to occur in the region 800-1000cm-147.With reference to this, the observed medium band at 1193cm-1 in FTIR is attributed to βC-H of thiophen ring. For the same mode the theoretical frequencies are:1370,1212cm-1 (mode nos:27,34). The harmonic wavenumbers:1066 and 862, 705cm-1 (mode nos:40 and 53,61) are designated as βHCS and τHCCS modes, respectively and theseassignments have considerable TED values (>50%).
In this work, it has been established well and the calculated wavenumbers in the range 3098-3016cm-1 (mode nos:2,6,8,10) are designated as νC-H modes of pentadiene moiety46, 47. For this mode, the corresponding vibrational bands are missing in the experimental spectra, which may be dueto isomer. Hence, the above assignments are justified with the help of TED values (>72%).The βC-H modes are attributed to the FTIR/FT-Raman bands present at 1404/1406cm-1 and for the same mode the harmonic frequencies are 1464,1437,1428,1409cm-1(mode nos: 21,23,25,26). This assignment is aggreable with literature 47in addition to TED results (>42%). The τCCCH vibrations are characterized by the FT-Raman/FTIR bands at 834,691/697cm-1,while the calculated wavenumbers are: 979, 958,831,825,689cm-1(mode nos:46,48,54,55,62) and also have considerable TED values (>42%).
NLO Property
The total dipole moment and first-order hyperpolarizability (β0) of MPPO is calculated by using B3LYP/6-311++G(d,p) basis set and are listed in Table 3.The calculated dipole moment and the mean first hyperpolarizability (β0) values are 0.7071 Debye and 10.2328x10-30 esu, respectively, and the values are presented in Table 3. Total hyperpolarizability of the title molecule is approximately twenty seven times greater than that of urea. The above results show that MPPO can be best material for NLO applications.
Table 3. The NLO measurements of MPPO.| Parameters | B3LYP/6-311++G(d,p) |
| Dipole moment ( μ ) Debye | |
| μx | -0.4885 |
| μy | 0.5112 |
| μz | 0.0001 |
| Μ | 0.7071 Debye |
| Polarizability ( α0) x10-30esu | |
| αxx | 418.01 |
| αxy | 4.69 |
| αyy | 237.33 |
| αxz | -0.01 |
| αyz | 0 |
| αzz | 113.8 |
| α0 | 0.69x10-30esu |
| Hyperpolarizability ( β0 ) x10-30esu | |
| βxxx | 284.46 |
| βxxy | 804.11 |
| βxyy | 107.07 |
| βyyy | 306.1 |
| βxxz | 0.02 |
| βxyz | -0.01 |
| βyyz | 0.06 |
| βxzz | 22.34 |
| βyzz | -0.42 |
| βzzz | 0.0044 |
| β0 | 10.23x10-30esu |
NBO Analysis
The NBO analysis is performed on MPPO using B3LYP/6-311++G(d,p) basis set. The E(2) energies and types of interactions are listed in Table 4.The EDs of the conjugated single as well as double bond of the aromatic/thiophene rings are 1.979& 1.606e/1.979&1.773e,respectively.This exhibits a strong delocalization inside the molecule MPPO. The non-bonding atoms transfer more energy to the acceptor, during the intra-molecular interactions. The LP(2) S5 and LP(2) O17 atoms transfer more energy (27.15 and 14.61 KJ/mol) to anti-bonding orbitals C3-C4 and C14-C16, respectively, when compare with σ-σ* transition for the same. These interactions causes a pronounced and moderate decrease of the lone pair orbitals occupancies; 1.581e and 1.886e, respectively, and there is a possibility for hyperconjugativeinteraction between them.The donor and acceptor interactions of πC22-C24→π*C23-C25(20.9 KJ/mol); πC27-C29→π*C23-C25(18.55KJ/mol) reveal the maximum hyperconjugative interaction in benzene ring of MPPO.
Table 4. The second order perturbation theory analysis of Fock Matrix in NBO basis for MPPO| Type | Donor NBO (i) | ED/e | Acceptor NBO (j) | ED/e | E(2) KJ/mol | E(j)-E(i) a.u. | F(i,j) a.u. | ||||||||
| σ-σ* | BD ( 1) C 1 - C 2 | 1.97397 | BD*( 1) C 1 - C 12 | 0.0274 | 3.73 | 1.22 | 0.06 | ||||||||
| BD*( 1) C 12 - C 14 | 0.01462 | 1.94 | 1.96 | 0.055 | |||||||||||
| π-π* | BD ( 2) C 1 - C 2 | 1.73778 | BD*( 2) C 3 - C 4 | 0.32831 | 16.62 | 0.27 | 0.06 | ||||||||
| BD*( 1) C 8 - H 10 | 0.00953 | 1.87 | 0.66 | 0.034 | |||||||||||
| BD*( 1) C 8 - H 11 | 0.00953 | 1.87 | 0.66 | 0.034 | |||||||||||
| BD*( 2) C 12 - C 14 | 0.17953 | 20.25 | 0.33 | 0.074 | |||||||||||
| σ-σ* | BD ( 1) C 1 - S 5 | 1.97486 | BD*( 1) C 4 - H 7 | 0.01311 | 2.76 | 1.22 | 0.052 | ||||||||
| BD*( 1) C 12 - H 13 | 0.01378 | 1.76 | 1.08 | 0.039 | |||||||||||
| σ-σ* | BD ( 1) C 1 - C 12 | 1.97917 | BD*( 1) C 12 - C 14 | 0.01462 | 1.53 | 1.96 | 0.049 | ||||||||
| BD*( 1) C 14 - H 15 | 0.01324 | 1.42 | 1.16 | 0.036 | |||||||||||
| BD*( 1) C 16 - C 18 | 0.05908 | 34.4 | 0.24 | 0.082 | |||||||||||
| BD*( 1) C 18 - H 19 | 0.01254 | 120.55 | 0.08 | 0.09 | |||||||||||
| BD*( 1) C 22 - C 24 | 0.02627 | 27.23 | 0.32 | 0.083 | |||||||||||
| BD*( 2) C 22 - C 24 | 0.36662 | 36.29 | 0.13 | 0.067 | |||||||||||
| σ-σ* | BD ( 1) C 2 - C 3 | 1.97199 | BD*( 1) C 1 - C 12 | 0.0274 | 4.38 | 1.19 | 0.065 | ||||||||
| BD*( 1) C 3 - C 4 | 0.01512 | 2.83 | 1.36 | 0.056 | |||||||||||
| BD*( 1) C 4 - H 7 | 0.01311 | 3.02 | 1.25 | 0.055 | |||||||||||
| σ-σ* | BD ( 1) C 2 - C 8 | 1.97883 | BD*( 1) C 3 - C 4 | 0.01512 | 1.28 | 1.32 | 0.037 | ||||||||
| σ-σ* | BD ( 1) C 3 - C 4 | 1.98319 | BD*( 1) C 4 - H 7 | 0.01311 | 1.34 | 1.3 | 0.037 | ||||||||
| π-π* | BD ( 2) C 3 - C 4 | 1.8072 | BD*( 2) C 3 - C 4 | 0.32831 | 0.65 | 0.28 | 0.012 | ||||||||
| σ-σ* | BD ( 1) C 3 - H 6 | 1.97779 | BD*( 1) C 3 - C 4 | 0.01512 | 1.33 | 1.21 | 0.036 | ||||||||
| σ-σ* | BD ( 1) C 4 - S 5 | 1.97845 | BD*( 1) C 1 - C 12 | 0.0274 | 4.22 | 1.18 | 0.063 | ||||||||
| σ-σ* | BD ( 1) C 4 - H 7 | 1.98573 | BD*( 1) C 3 - C 4 | 0.01512 | 1.3 | 1.23 | 0.036 | ||||||||
| BD*( 1) C 16 - C 18 | 0.05908 | 13.02 | 0.08 | 0.03 | |||||||||||
| BD*( 1) C 22 - C 24 | 0.02627 | 7.54 | 0.16 | 0.031 | |||||||||||
| σ-σ* | BD ( 1) C 8 - H 9 | 1.98812 | BD*( 1) C 12 - C 14 | 0.01462 | 3.95 | 1.87 | 0.077 | ||||||||
| BD*( 1) C 16 - O 17 | 0.01724 | 4.38 | 1.27 | 0.067 | |||||||||||
| BD*( 1) C 24 - H 28 | 0.02271 | 2.98 | 1.1 | 0.051 | |||||||||||
| BD*( 2) C 27 - C 29 | 0.33144 | 2.97 | 3.85 | 0.104 | |||||||||||
| BD*( 1) C 27 - H 31 | 0.01409 | 2.52 | 4.81 | 0.098 | |||||||||||
| σ-σ* | BD ( 1) C 8 - H 10 | 1.9793 | BD*( 1) C 22 - C 24 | 0.02627 | 74.35 | 0.12 | 0.086 | ||||||||
| σ-σ* | BD ( 1) C 12 - H 13 | 1.96197 | BD*( 1) C 14 - H 15 | 0.01324 | 1.05 | 0.96 | 0.029 | ||||||||
| BD*( 1) C 14 - C 16 | 0.05431 | 5.72 | 1.06 | 0.07 | |||||||||||
| σ-σ* | BD ( 1) C 12 - C 14 | 1.98129 | BD*( 1) C 1 - C 12 | 0.0274 | 4.63 | 1.25 | 0.068 | ||||||||
| BD*( 1) C 14 - C 16 | 0.05431 | 2.42 | 1.28 | 0.05 | |||||||||||
| BD*( 1) C 16 - C 18 | 0.05908 | 8.93 | 0.27 | 0.044 | |||||||||||
| π-π* | BD ( 2) C 12 - C 14 | 1.81895 | BD*( 2) C 16 - O 17 | 0.27328 | 21.41 | 0.29 | 0.072 | ||||||||
| σ-σ* | BD ( 1) C 14 - H 15 | 1.97581 | BD*( 1) C 1 - C 12 | 0.0274 | 7.09 | 1.02 | 0.076 | ||||||||
| BD*( 1) C 16 - O 17 | 0.01724 | 3.76 | 1.16 | 0.059 | |||||||||||
| σ-σ* | BD ( 1) C 14 - C 16 | 1.9799 | BD*( 1) C 12 - H 13 | 0.01378 | 1.96 | 1.09 | 0.041 | ||||||||
| BD*( 1) C 12 - C 14 | 0.01462 | 2.04 | 1.91 | 0.056 | |||||||||||
| BD*( 1) C 16 - C 18 | 0.05908 | 5.72 | 0.19 | 0.03 | |||||||||||
| BD*( 1) C 18 - H 19 | 0.01254 | 4.58 | 0.03 | 0.011 | |||||||||||
| σ-σ* | BD ( 1) C 16 - O 17 | 1.99368 | BD*( 1) C 16 - C 18 | 0.05908 | 2.76 | 0.57 | 0.036 | ||||||||
| π-π* | BD ( 2) C 16 - O 17 | 1.96036 | BD*( 2) C 12 - C 14 | 0.17953 | 4.25 | 0.43 | 0.039 | ||||||||
| BD*( 2) C 18 - C 20 | 0.13135 | 4.57 | 0.4 | 0.039 | |||||||||||
| σ-σ* | BD ( 1) C 16 - C 18 | 1.98102 | BD*( 1) C 12 - C 14 | 0.01462 | 1.59 | 1.91 | 0.049 | ||||||||
| BD*( 1) C 18 - H 19 | 0.01254 | 6.35 | 0.03 | 0.012 | |||||||||||
| BD*( 1) C 20 - H 21 | 0.01242 | 1.68 | 1.09 | 0.038 | |||||||||||
| σ-σ* | BD ( 1) C 18 - H 19 | 1.97184 | BD*( 1) C 16 - O 17 | 0.01724 | 4.69 | 1.15 | 0.066 | ||||||||
| BD*( 1) C 20 - H 21 | 0.01242 | 1.26 | 0.94 | 0.031 | |||||||||||
| BD*( 1) C 20 - C 22 | 0.02819 | 7.7 | 1 | 0.078 | |||||||||||
| σ-σ* | BD ( 1) C 18 - C 20 | 1.98216 | BD*( 1) C 16 - C 18 | 0.05908 | 14.3 | 0.27 | 0.056 | ||||||||
| BD*( 1) C 18 - H 19 | 0.01254 | 8.42 | 0.11 | 0.028 | |||||||||||
| BD*( 1) C 20 - C 22 | 0.02819 | 3.69 | 1.23 | 0.06 | |||||||||||
| π-π* | BD ( 2) C 18 - C 20 | 1.84033 | BD*( 2) C 16 - O 17 | 0.27328 | 20.32 | 0.29 | 0.07 | ||||||||
| σ-σ* | BD ( 1) C 20 - H 21 | 1.97149 | BD*( 1) C 16 - C 18 | 0.05908 | 73.62 | 0.05 | 0.056 | ||||||||
| BD*( 1) C 22 - C 24 | 0.02627 | 40.86 | 0.13 | 0.065 | |||||||||||
| σ-σ* | BD ( 1) C 20 - C 22 | 1.97691 | BD*( 1) C 16 - C 18 | 0.05908 | 9.02 | 0.2 | 0.038 | ||||||||
| BD*( 1) C 22 - C 23 | 0.02207 | 2.06 | 1.22 | 0.045 | |||||||||||
| BD*( 1) C 22 - C 24 | 0.02627 | 32.81 | 0.27 | 0.085 | |||||||||||
| BD*( 2) C 22 - C 24 | 0.36662 | 7.71 | 0.08 | 0.025 | |||||||||||
| σ-σ* | BD ( 1) C 22 - C 23 | 1.97392 | BD*( 1) C 20 - C 22 | 0.02819 | 2.17 | 1.17 | 0.045 | ||||||||
| BD*( 2) C 22 - C 24 | 0.36662 | 120.04 | 0.1 | 0.106 | |||||||||||
| σ-σ* | BD ( 1) C 22 - C 24 | 1.9728 | BD*( 1) C 23 - H 26 | 0.0143 | 3.64 | 1.12 | 0.057 | ||||||||
| BD*( 1) C 24 - C 27 | 0.01547 | 2.59 | 1.27 | 0.051 | |||||||||||
| π-π* | BD ( 2) C 22 - C 24 | 1.5862 | BD*( 2) C 18 - C 20 | 0.13135 | 13.05 | 0.29 | 0.059 | ||||||||
| BD*( 2) C 23 - C 25 | 0.31148 | 20.9 | 0.29 | 0.071 | |||||||||||
| BD*( 2) C 27 - C 29 | 0.33144 | 2.84 | 3.47 | 0.09 | |||||||||||
| σ-σ* | BD ( 1) C 23 - C 25 | 1.97917 | BD*( 1) C 4 - H 7 | 0.01311 | 3.66 | 1.28 | 0.061 | ||||||||
| BD*( 1) C 20 - C 22 | 0.02819 | 3.26 | 1.21 | 0.056 | |||||||||||
| BD*( 1) C 22 - C 23 | 0.02207 | 4.39 | 1.27 | 0.067 | |||||||||||
| σ-σ* | BD ( 1) C 23 - H 26 | 1.98069 | BD*( 1) C 22 - C 23 | 0.02207 | 0.69 | 1.07 | 0.024 | ||||||||
| σ-π* | BD ( 1) C 24 - C 27 | 1.97888 | BD*( 2) C 3 - C 4 | 0.32831 | 3.77 | 0.9 | 0.056 | ||||||||
| BD*( 1) C 4 - H 7 | 0.01311 | 6.05 | 1.47 | 0.084 | |||||||||||
| BD*( 1) C 16 - O 17 | 0.01724 | 4.9 | 1.55 | 0.078 | |||||||||||
| BD*( 1) C 20 - C 22 | 0.02819 | 4.41 | 1.39 | 0.07 | |||||||||||
| σ-σ* | BD ( 1) C 24 - H 28 | 1.97705 | BD*( 1) C 16 - C 18 | 0.05908 | 27.9 | 0.02 | 0.023 | ||||||||
| BD*( 1) C 22 - C 23 | 0.02207 | 4.86 | 1.05 | 0.064 | |||||||||||
| BD*( 1) C 22 - C 24 | 0.02627 | 27.94 | 0.1 | 0.048 | |||||||||||
| σ-π* | BD ( 1) C 25 - C 29 | 1.97941 | BD*( 2) C 3 - C 4 | 0.32831 | 4.09 | 0.69 | 0.052 | ||||||||
| BD*( 1) C 12 - C 14 | 0.01462 | 10.22 | 1.94 | 0.126 | |||||||||||
| BD*( 1) C 16 - O 17 | 0.01724 | 9.45 | 1.34 | 0.101 | |||||||||||
| BD*( 1) C 23 - H 26 | 0.0143 | 6.31 | 1.13 | 0.075 | |||||||||||
| BD*( 1) C 24 - H 28 | 0.02271 | 8.21 | 1.17 | 0.087 | |||||||||||
| σ-σ* | BD ( 1) C 25 - H 30 | 1.98016 | BD*( 1) C 4 - H 7 | 0.01311 | 3.33 | 1.1 | 0.054 | ||||||||
| BD*( 1) C 22 - C 23 | 0.02207 | 5.6 | 1.08 | 0.069 | |||||||||||
| BD*( 1) C 24 - H 28 | 0.02271 | 3.22 | 1 | 0.051 | |||||||||||
| BD*( 1) C 29 - H 32 | 0.01371 | 2.72 | 2.61 | 0.075 | |||||||||||
| σ-π* | BD ( 1) C 27 - C 29 | 1.98062 | BD*( 2) C 3 - C 4 | 0.32831 | 4.13 | 0.69 | 0.052 | ||||||||
| BD*( 1) C 12 - C 14 | 0.01462 | 9.11 | 1.94 | 0.119 | |||||||||||
| BD*( 1) C 16 - O 17 | 0.01724 | 9.4 | 1.34 | 0.1 | |||||||||||
| BD*( 2) C 27 - C 29 | 0.33144 | 16.2 | 3.92 | 0.245 | |||||||||||
| π-π* | BD ( 2) C 27 - C 29 | 1.64275 | BD*(2) C 23 - C 25 | 0.31148 | 18.55 | 0.29 | 0.066 | ||||||||
| σ-σ* | BD ( 1) C 27 - H 31 | 1.98023 | BD*(1) C 4 - H 7 | 0.01311 | 3.3 | 1.08 | 0.053 | ||||||||
| BD*(1) C 24 - H 28 | 0.02271 | 5.55 | 0.99 | 0.066 | |||||||||||
| σ-π* | BD ( 1) C 29 - H 32 | 1.98091 | BD*( 2) C 3 - C 4 | 0.32831 | 4.65 | 0.48 | 0.046 | ||||||||
| BD*( 1) C 12 - C 14 | 0.01462 | 26.51 | 1.73 | 0.191 | |||||||||||
| BD*( 1) C 16 - O 17 | 0.01724 | 16.8 | 1.13 | 0.123 | |||||||||||
| BD*( 1) C 24 - H 28 | 0.02271 | 9.57 | 0.96 | 0.086 | |||||||||||
| BD*( 2) C 27 - C 29 | 0.33144 | 23.61 | 3.71 | 0.288 | |||||||||||
| BD*( 1) C 27 - H 31 | 0.01409 | 34.65 | 4.66 | 0.359 | |||||||||||
| n-σ* | LP ( 1) S 5 | 1.98351 | BD*( 1) C 3 - C 4 | 0.01512 | 2.25 | 1.32 | 0.049 | ||||||||
| n-π* | LP ( 2) S 5 | 1.58098 | BD*( 2) C 3 - C 4 | 0.32831 | 27.15 | 0.24 | 0.074 | ||||||||
| n-σ* | LP ( 1) O 17 | 1.969 | BD*( 1) C 14 - C 16 | 0.05431 | 2.03 | 1.23 | 0.045 | ||||||||
| BD*( 1) C 16 - C 18 | 0.05908 | 5.78 | 0.22 | 0.032 | |||||||||||
| BD*( 1) C 24 - H 28 | 0.02271 | 2.57 | 1.16 | 0.049 | |||||||||||
| n-π* | LP ( 2) O 17 | 1.88598 | BD*( 1) C 14 - C 16 | 0.05431 | 14.61 | 0.81 | 0.098 | ||||||||
| BD*( 1) C 24 - H 28 | 0.02271 | 2.64 | 0.74 | 0.04 | |||||||||||
| π*-σ* | BD*( 2) C 1 - C 2 | 0.39784 | BD*( 1) C 12 - C 14 | 0.01462 | 8.28 | 33.56 | 1.036 | ||||||||
| BD*( 1) C 16 - O 17 | 0.01724 | 8.11 | 32.96 | 1.013 | |||||||||||
| BD*( 2) C 27 - C 29 | 0.33144 | 21.31 | 35.54 | 1.286 | |||||||||||
| BD*( 1) C 27 - H 31 | 0.01409 | 8.56 | 36.49 | 1.099 | |||||||||||
| BD*( 1) C 29 - H 32 | 0.01371 | 4.11 | 34.39 | 0.74 | |||||||||||
| π*-π* | BD*( 2) C 3 - C 4 | 0.32831 | BD*( 2) C 16 - O 17 | 0.27328 | 0.71 | 0.02 | 0.005 | ||||||||
| π*-σ* | BD*( 2) C 12 - C 14 | 0.17953 | BD*( 1) C 29 - H 32 | 0.01371 | 2.9 | 2.02 | 0.22 | ||||||||
| π*-π* | BD*( 2) C 16 - O 17 | 0.27328 | BD*( 2) C 12 - C 14 | 0.17953 | 35.93 | 0.05 | 0.077 | ||||||||
| BD*( 2) C 18 - C 20 | 0.13135 | 62.96 | 0.02 | 0.074 | |||||||||||
| π*-π* | BD*( 2) C 18 - C 20 | 0.13135 | BD*( 2) C 27 - C 29 | 0.33144 | 1.45 | 3.19 | 0.126 | ||||||||
| π*-π* | BD*( 2) C 22 - C 24 | 0.36662 | BD*( 2) C 3 - C 4 | 0.32831 | 5.26 | 0.58 | 0.084 | ||||||||
| BD*( 1) C 16 - O 17 | 0.01724 | 3.86 | 1.23 | 0.141 | |||||||||||
| BD*( 2) C 23 - C 25 | 0.31148 | 6.92 | 0.62 | 0.101 | |||||||||||
| BD*( 1) C 24 - H 28 | 0.02271 | 3.37 | 1.06 | 0.121 | |||||||||||
| π*-π* | BD*( 2) C 23 - C 25 | 0.31148 | BD*( 2) C 27 - C 29 | 0.33144 | 6.83 | 3.19 | 0.232 | ||||||||
| π*-σ* | BD*( 2) C 27 - C 29 | 0.33144 | BD*( 1) C 27 - H 31 | 0.01409 | 367.87 | 0.96 | 1.273 | ||||||||
Homo-Lumo Analysis
Molecular orbital and their properties like energy are very useful to the physicists and chemists 44. The HOMO acts as an electron donor and the LUMO is the electron acceptor, and the gap between HOMO and LUMO characterizes the molecular chemical stability. The energy gap between the HOMO and the LUMO MOs is a critical parameter in determining molecular electrical transport properties because it is a measure of electron conductivity. Figure 4 shows, the HOMO-LUMO plots of MPPO. The HOMO is located over the thiophen ring and partly on CH3 group. The LUMO located all over the molecule except methyl group, since methyl group has large electronegativity. The energy gap of MPPO molecule is calculated as 3.4327 eV in gas phase using B3LYP/6-311++G(d,p) basis set.HOMO implies the energy of about -6.0551 eV and LUMO energy is -2.6224 eV. The various physico-chemical parameters are listed in Table 5 and the frontier molecular orbital energies are also listed in Table 6.
Figure 4.The frontier molecular orbital of MPPO
| Parameters | Values |
| HOMO | -6.055 eV |
| LUMO | -2.622 eV |
| Energy gap | 3.432 eV |
| Ionization potential (IP) | 6.055 eV |
| Electron affinity (EA) | 2.622 eV |
| Electrophilicity Index (ω) | 2.741 |
| Chemical Potential (µ) | 4.338 |
| Electronegativity (χ) | 4.338 |
| Hardness (η) | -3.432 |
| Softness (S) | 6.866 |
| Occupancy | Orbital energies (a.u) | Orbital energies (eV) | Kinetic energies (a.u) |
| O52 | -0.269 | -7.343 | 1.118 |
| O53 | -0.252 | -6.858 | 2.314 |
| O54 | -0.246 | -6.708 | 1.429 |
| O55 | -0.241 | -6.569 | 1.348 |
| O56 | -0.235 | -6.405 | 1.388 |
| V57 | -0.097 | -2.646 | 1.398 |
| V58 | -0.051 | -1.397 | 1.289 |
| V59 | -0.025 | -0.694 | 1.244 |
| V60 | 0.016 | -0.441 | 1.358 |
| V61 | 0.013 | -0.373 | 0.251 |
UV-Vis Spectra
The electronic spectra of the title moleculeis calculated using TD-DFT/B3LYP/6-311++G(d,p) basis set. The electronic transitions, positions of experimental absorption peaks, calculated absorption peaks (λmax), vertical excitation energies, oscillator strengths (f) are listed in Table 7. The observed and UV-Vis absorption maximum of MPPO are shown in Figure 5 In this study, the electronic transition is calculated at three excited states (ES) like as ES1, ES2 and ES3, respectively,in which ES1 appeared at 421.19nm (2.9436eV), ES2 at 398.42 nm (3.1119eV) and ES3 lies at 343.32nm (3.6113eV). In ES3 state there are three transitions from HOMO 64 to Lumo 68, HOMO66 to LUMO68 and HOMO 67 to LUMO 69. The molecule MPPO has sulphur and oxygen as lone pairs, hence π-π* transition is also possible.The electronic absorption calculation shows that the visible absorption maximafor MPPO corresponds to the electron transition between frontier orbitals such as transition from HOMO to LUMO. It should be mentioned here that the low energy absorptions is found at 303 nm and 352 nm belong to the dipole allowed σ-σ* and π-π* transition from HOMO to LUMO, respectively. The intense bands calculated at 398nm can be assigned to high delocalization of π-electrons in the thiophen moiety of MPPO. Similarly the intense band at 343nm can be distributed to the excitation of σ-electrons which are localized on the whole molecular system.
Table 7. The electronic transitions of MPPO| Calculated at B3LYP/6-311++G(d,p) | Oscillator Strength | Calculated Band gap(ev/nm) | Experimental Type Band gap (ev/nm) |
| Excited State-1 | Singlet-A(f=0.0000) | 2.9436 eV/421.19 nm | |
| 65 -> 68 | 0.68512 | 4.164218 | |
| Excited State-2 | Singlet-A(f=0.6489) | 3.6113 eV/343.32 nm | 352 π–π* |
| 67 -> 68 | 0.64129 | 3.432542 | |
| Excited State-3 | Singlet-A(f=0.0507) | 3.6113 eV/343.32 nm | 303 π –π* |
| 64 -> 68 | 0.28196 | 4.289929 | |
| 66 -> 68 | 0.58917 | 3.960688 | |
| 67 -> 69 | 0.13934 | 4.689976 |
Figure 5.The combined theoretical and experimental UV-Visible spectra of MPPO
MEP Analysis
MEP indicates the net electrostatic effect produced at that point by the total charge distribution (nuclei + electron) of the molecule and correlates with dipole moments, electronegativity, partial charges and chemical reactivity of the molecules. It provides a visual method to understand the relative polarity of the molecule. The different values of the electrostatic potential represented by different colors; red represents the regions of the most negative electrostatic potential, blue represents the regions of the most positive electrostatic potential and green represents the regions of zero potential.The MEP is calculated with B3LYP/6-311++G(d,p) basis set and the MEP plot is shown in Figure 6.
Figure 6.The molecular electrostatic potential map of MPPO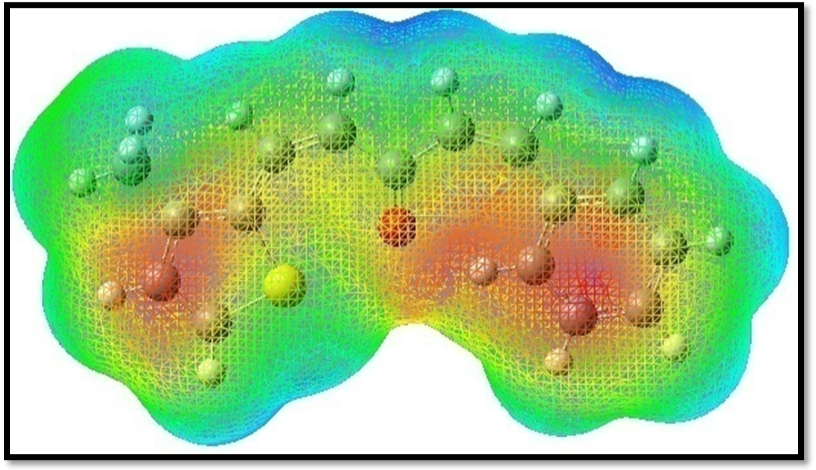
The negative electrostatic potential corresponds to an attraction of the proton by the aggregate ED in the molecule (shades of red), while all the hydrogen atoms have positive electrostatic potential corresponds to the repulsion of the proton by the atomic nuclei (shades of blue). The MEP map shows that the negative region is mainly localized on oxygen and sulphur atoms while the positive region is localized on the surface of all the hydrogen atoms in MPPO. These regions indicate the active charge sites of the molecule. A region of zero potential envelopes the π-system of the aromatic rings 48.
Mulliken Charge Analysis
The calculation of atomic charges plays an important role in the application of quantum mechanical calculation to molecular system 49.The Mulliken charges of MPPO was calculated at B3LYP/6-311++G(d,p) level of theory and are listed in Table 8. The mulliken atomic charge plot is shown in Figure 7. The carbon atom (C22) has more positive charge (0.9652 a.u), which isfused linkage with highelectronegative carbonyl group. Similarly,the methyl group carbon atom C8 has maximum negative charge (-0.9825 a.u), sinceit isattached with thiophene ring. In MPPO all the hydrogen atoms have positive charge.
Table 8. The Mulliken atomic charges of MPPO| Atoms | Charges | Atoms | Charges |
| 1C | 0.519 | 17O | -0.2251 |
| 2C | 0.0009 | 18C | -0.3645 |
| 3C | -0.2145 | 19H | 0.1716 |
| 4C | -0.0636 | 20C | -0.2478 |
| 5S | -0.5428 | 21H | 0.1456 |
| 6H | 0.1464 | 22C | 0.9651 |
| 7H | 0.2521 | 23C | 0.1338 |
| 8C | -0.9824 | 24C | -0.7922 |
| 9H | 0.1601 | 25C | -0.3845 |
| 10H | 0.1602 | 26H | 0.1524 |
| 11H | 0.1602 | 27C | -0.1456 |
| 12C | 0.219 | 28H | 0.1564 |
| 13H | 0.1608 | 29C | -0.1516 |
| 14C | -0.1696 | 30H | 0.1636 |
| 15H | 0.1724 | 31H | 0.1782 |
| 16C | 0.1067 | 32H | 0.1589 |
Thermodynamic Properties
The various thermodynamic parameter are computed by B3LYP/6-311++G(d,p) basis set and are listed in Table 9. The thermodynamic functions like heat capacity (C0p,m), entropy (S0m) and enthalpy changes (ΔH0m) are obtained from the theoretical harmonic frequencies. From Table 10, it can be seen that these thermodynamic functions are increasing with temperature ranging from 100 to 1000 K due to the fact that the molecular vibrational intensities increase with temperature 51. The correlation equations between entropy, heat capacity, enthalpy changes and temperatures were fitted by quadratic formulas and the correspondingare fitting factors (R2) for those thermodynamic properties are 0.9999, 0.9992 and 0.9994, respectively. The corresponding fitting equations are as follows and the correlation graphics are shown in Figure 8.
S0m = 0.98075 + 0.00414T - 3.65824 x 10-5 T2 (R2 = 0.9999)
C0p,m = 6.04475 + 0.02552T +2.25471x 10-5 T2 (R2 = 0.9992)
ΔH0m = 4.00201 + 0.169T + 1.49276x 10-5 T2 (R2 = 0.9994)
Table 9. The calculated total energy (a.u), zero point vibrational energies (Kcal/mol), rotational constants (GHz) and entropy (cal/mol K-1) for MPPO| Parameters | B3LYP/6-311++G(d,p) |
| Total Energies | -1091.71 |
| Zero-point Energy | 158.198 (Kcal/Mol) |
| Rotational constants (GHZ) | 1.013 |
| 0.156 | |
| 0.135 | |
| Entropy | |
| Total | 135.128 |
| Translational | 42.498 |
| Rotational | 33. 969 |
| Vibrational | 58.661 |
| T (K) | S (J/mol.K) | Cp (J/mol.K) | ddH (kJ/mol) |
| 100 | 376.62 | 119.6 | 8.02 |
| 200 | 481.8 | 194.81 | 23.62 |
| 298.15 | 575.09 | 278.44 | 46.82 |
| 300 | 576.82 | 280.01 | 47.34 |
| 400 | 668.56 | 359.67 | 79.42 |
| 500 | 756.18 | 425.73 | 118.81 |
| 600 | 838.65 | 478.52 | 164.12 |
| 700 | 915.71 | 520.88 | 214.17 |
| 800 | 987.59 | 555.43 | 268.04 |
| 900 | 1054.72 | 584.04 | 325.06 |
| 1000 | 1117.53 | 608.01 | 384.69 |
Figure 8.The thermodynamic properties of MPPO at different temperatures
The above data can be used to compute other thermodynamic energies according to the well-known relationships of thermodynamic functions and to predict the directions of chemical reactions 52. All the thermodynamic calculations were done in gas phase and they could not be used in solution.
Conclusion
A complete vibrational analysis had been carried out for the first time to the molecule MPPO. The bond parameters and vibrational wavenumbers agreed well with experimental results. The first order hyperpolarizability (β0 = 10.2328x10-30esu) of MPPO was calculated and found to be twenty seven times greater than that of urea and hence the molecule had moderate NLO activity. The considerabledecrease of the lone pair orbital occupancy (1.581e) of LP(2)S5was due to more E(2) energy transfer to anti-bonding orbital C3-C4. The Homo-Lumo energy gap was calculated about 3.4327 eV, which explain the eventual charge transfer occur within the molecule and also enhanced the biological activity. The recorded UV-Vis. spectral values agreed with calculated values. The λmax (352) was assigned to π-π* type. Furthermore, the MEP and Mulliken atomic charges had been calculated and also plotted. The good correlations between the statistical thermodynamics and temperature were also established.
References
- 1.Li R, G L Kenyon, F E Cohen, Chen X, Gong B et al. (1995) . In Vitro Antimalarial Activity of Chalcones and Their Derivatives”, J.Med. Chem 38, 5031-5037.
- 2.J F Ballesteros, M J Sanz, Ubeda A, M A, Iborra S et al. (1995) Synthesis and Pharmacological Evaluation of 2'-Hydroxychalcones and Flavones as Inhibitors of Inflammatory Mediators Generation”. , J. Med.Chem 38, 2794-2797.
- 3.L W Wattenberg, J B Coccia, A R Galhaith, Galbraith.Inhibition of carcinogen-induced pulmonary and mammary carcinogenesis by chalcone administered subsequent to carcinogen exposure”. , Cancer Lett 83, 165-169.
- 4.A T Dinkova-Kostova, Abeygunawardana C, Talalay P. (1998) Chemoprotective Properties of Phenylpropenoids, Bis(benzylidene)cycloalkanones, and Related Michael Reaction Acceptors:. Correlation of Potencies as Phase 2 Enzyme Inducers and Radical Scavengers” , J. Med. Chem 41, 5287-5296.
- 5.Satomi Y. (1993) Inhibitory effects of 3′-methyl-3-hydroxy-chalcone on proliferation of human malignant tumor cells and on skin carcinogenesis”. , Int. J. Cancer 55, 506-514.
- 6.Yit C C, N P Das. (1994) Cytotoxic effect of butein on human colon adenocarcinoma cell proliferation”. , Cancer Lett 82, 65-72.
- 7.Dimmock J R, Kandepu N M, Hetherington M, J W Quail, Pugazhenthi U et al. (1998) . , J. Med. Chem 41, 1014-1026.
- 8.Mantas A, Deretey E, Ferretti F H, Estrada M R, Csizmadia. (2000) Structural analysis of flavonoids with anti-HIV activity”,J.Mol.Struct. 504. 171-179.
- 9.Sivakumar M, Phrabusreeneivasan S, Kumar V, Doble M, Bioorg. (2007) . , Med. Chem. Lett 17(10), 3169.
- 10.Liu X, M L Go. (2006) . , Antiproliferative Properties of Piperidinylchalcones”, Bioorg. Med. Chem 14, 153-163.
- 11.Arulkumaran R, Sundararajan R, Vanangamudi G, Subramanian M, Ravi K et al. (2010) . , J. Chem 3(1), 82-98.
- 12.Deng J, Sanchez T, Lalith Q A M. (2007) Discovery of structurally diverse HIV-1integrase inhibitors based on a chalcone pharmacophore’’. , Bioorg. Med.Chem 15(14), 4985-5002.
- 14.Thirunarayanan G, Surya S, Srinivasan S, Vanangamudi G, Sathyendiran. (2010) V.,“Synthesis and insect antifeedant activities of some substituted styryl 3,4-dichlorophenyl ketones”, Spectrochim. Acta 75A. 152-156.
- 15.Wu X, Wilairat P, M L Go. (2002) Antimalarial activity of ferrocenyl chalcones”. , Bioorg. Med. Chem. Lett 12, 2299-2302.
- 16.Fouda M F R, Abd-Elzaher M M, Abdelsamaia R A, Labib A A. (2007) On the medicinal chemistry of ferrocene”. , Appl. Organometal. Chem 21, 613-625.
- 17.Wu X, Tiekink E R T, Kostetski I, Kocherginsky N, Tan A L C Khoo et al.. , Antiplasmodial Activity of Ferrocenyl Chalcones:Investigations into the Role of Ferrocene”, Eur. J. Pharm. Sci 27, 2006-175.
- 18.Cianci J, J B Baell, B L Flynn, Gable W, J A Mould et al.Synthesis and biological evaluation of chalcones as inhibitors of the voltage-gated potassium channel Kv1.3”, Bio. , Med. Chem.Lett 18, 2008-2055.
- 19.Chidankumar C S, Govindarasu K, Hoong-Kun F, Kavitha E, Chandraju S et al. (2015) Synthesis, molecular structure, spectroscopic characterization and quantum chemical calculation studies of (2E)-1-(5-chlorothiophen-2-yl)-3-(2,3,4-trimethoxyphenyl)prop-2-en-1-one”. , J. Mol. Struct 1085, 63-77.
- 20.Karunakaran V, Balachandran V. (2013) Experimental and computational study on molecular structure, natural bond orbital and natural hybrid orbital analysis of non-linear optical material trans-3-(o-hydroxyphenyl-1-phenyl)-2-propen-1-one”. , J. Mol. Struct 1053, 66-78.
- 22.H B Schlegel. (1982) Optimization of equilibrium geometries and transition structures”. , J.Comput. Chem 3, 214-218.
- 24.Vanchinathan K, Bhagavannarayana G, Muthu K, Meenakshisundaram. (2011) S.P.,“Synthesis, crystal growth and characterization of 1,5-diphenylpenta-1,4-dien-3-one: An organic crystal,”. , Physica B 406, 4195-4199.
- 25.Pasterny K, Wrzalik R, Kupka T, Pasterna G. (2002) Theoretical and experimental vibrational studies on liquid thiophene and its acetonitrile solution”. , J. Mol.Struct 614, 297-304.
- 26.Radom L. (1993) Scaling Fators for obtaining vibrational Frequencies and zero-point energy from HF/6-31G* and mp2/6-31G*, Harmonic Frequencies”. , Instrum. J.Chem 33, 345-350.
- 27.Socrates G. (2001) Infrared and Raman characteristic group frequencies-Tables and. Charts”, 3rd Ed.,John Wiley & sons, Ltd , Chichester .
- 28.D L Vein, N B Colthup, W G Fateley, J G Grasselli. (1991) The Hand book of Infrared and Raman characteristic frequencies of organic molecules”,Academic Press,San Diego.
- 29.Druz˙bicki K, Mikuli E, M D Ossowska-Chru´sciel. (2010) Experimental (FT-IR, FT-RS) and theoretical (DFT) studies of vibrational dynamics and molecular structure of 4-n-pentylphenyl-4′-n-octyloxythiobenzoate (8OS5)”. , Vib. Spect 52, 54-62.
- 30.D N Satyanarayana. (2004) Vibrational Spectroscopy-Theory and Applications” New Delhi:New. Age International (P) Ltd. Pub., 2nd Ed .
- 31.Balachandran V, Janaki A, Nataraj A. (2014) Theoretical investigations on molecular structure, vibrational spectra,HOMO. LUMO, NBO analysis and hyperpolarizability calculations ofthiophene-2-carbohydrazide”, Spectrochim. Acta 118 A 321-330.
- 32.H M Badawi. (2009) Structural stability, C–N internal rotations and vibrational spectral analysis of non-planar phenylurea and phenylthiourea”, Spectrochim. Acta 72 A. 523-527.
- 33.G D Fleming, Koch R, Vallete Campos, M. (2006) Theoretical study of the syn and anti thiophene-2-aldehyde conformers using density functional theory and normal coordinate analysis”. , Spectrochim. Acta. 65 A 935-945.
- 34.Unal A, Eren B. (2013) . FT-IR, dispersive Raman, NMR, DFT and antimicrobial activity studies on 2-(Thiophen-2-yl)-1H-benzo[d]imidazole”, Spectrochim. Acta 114 A 129-136.
- 35.Tzeng W B, Narayanan K, Lin J L, Tung C C. (1998) Structures and vibrations of o-methylaniline in the S0 and S1 states studied by ab initio calculations and resonant two-photon ionization spectroscopy,” Spectrochim. Acta 55A. 153-162.
- 36.D A Kleinman. (1962) Nonlinear Dielectric Polarization in Optical Media,” Phy. Rev.126. 1977-1979.
- 38.N B Colthup, L H Daly, S E Wiberley. (1990) Introduction to Infrared and Raman Spectroscopy”,Academic Press,NewYork.
- 39.Sorates G. (1990) Infrared characteristic Group frequencies”, Wiley, inter science Pub.,New. , York
- 40.Erdogdu Y, Gulluoğlu M T. (2009) . Analysis of Vibrational Spectra of 2 and 3-Methylpiperidine Based on Density Functional Theory Calculations”, Spectrochim. Acta 74A 162-167.
- 41.Krishnakumar V, Ramasamy R. (2007) Vibrational spectra and structure of 5,6-diamino uracil and 5,6-dihydro-5-methyl uracil by density functional theory calculations”, Spectrochim. Acta 66A. 503-511.
- 42.M K Subramanian, P M Anbarasan, Ilangovan V, S M Babu. (2008) FT-IR, NIRFT-Raman and Gas Phase Infrared Spectra of 3-Aminoacetophenone by Density Functional Theory and Ab Initio Hartree–Fock Calculations”. , Spectrochim.Acta 71 A 59-67.
- 43.Varsanyi G. (1973) Assignments for Vibrational Spectra of Seven Hundred Benzene Derivatives”. Vol.1 and 2, Academic Kiado , Budapest .
- 44.Furic K, Mohacek V, Bonifacic M, Štefanic I. (1992) Raman spectroscopic study of H2O and D2O water solutions of glycine”. , J. Mol. Struct 267, 39-44.
- 45.A E Reed, L A Curtiss, Weinhold F. (1988) Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint”. , Chem. Rev 88, 899-926.
- 46.Varsanyi G. (1974) Assignments of Vibrational spectra of seven Hundred Benzene derivatives”,Wiley,New York.
- 47.Jag M. (2001) Organic Spectroscopy-Principle and Applications”, 2nd Ed.,Narosa Pub.House. , New Delhi
- 48.Fukui K, Yonezawa T, Shingu H. (1952) A Molecular Orbital Theory of Reactivity in Aromatic Hydrocarbons”. , J. Chem. Phys 20, 722-725.
- 49.V P Gupta, Sharma A, Virdi V, V J Ram. (2006) Structural and spectroscopic studies on some chloropyrimidine derivatives by experimental and quantum chemical methods”, Spectrochim. Acta 64A. 57-67.
Cited by (1)
This article has been cited by 1 scholarly work according to:
Citing Articles:
Journal of new developments in Chemistry (2016) OpenAlex

