Laryngeal Tissue Engineering using Rabbit Adipose Derived Stem Cells in Fibrin: A Pre-Clinical Model
Abstract
Vocal fold scarring is a clinical problem without reliable treatment. Tissue engineering of a vocal fold replacement is an exciting potential treatment for vocal fold scars that involve multiple layers of the vocal fold. Human adipose-derived stem cells (ASC) were previously used to produce a promising vocal fold cover layer replacement. However, relevant in vivo studies are needed before human application, and implanting the human cells in animal larynges would introduce significant risk and data confounding. We therefore report here the development of a construct based on rabbit ASC with the potential for use in pre-clinical implantation studies. Rabbit ASC were isolated and cultured in a three-dimensional fibrin matrix to create an implantable construct resembling the vocal fold mucosa. Key differences between the human cell and the rabbit cell models are highlighted.
Author Contributions
Academic Editor: Ioannis Chatzistefanou, Aristotle University of Thessaloniki (A.U.Th), Department of Maxillofacial Surgery, G. Papanikolaou General Hospital.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2015 Travis Shiba, et al
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
No conflict of interests. This material is based upon work supported by the Department of Veteran Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development (Jennifer Long and Jordan Hardy), VA Career Development Award IK2BX001944 (Jennifer Long), and the American Academy of Otolaryngology AAO-HNSF Resident Research Award (Travis Shiba).
Citation:
Introduction
Translating in vitro models into pre-clinical development is a significant hurdle in the advancement of tissue-engineered therapies. Potential therapies are typically developed in vitro, and then must be vetted in a relevant animal model before beginning human clinical trials. For tissue-engineered organ replacements, this means developing a species-specific implant. Construct design for preclinical animal trials may differ from both the initial in vitro product and the eventual human implant.
For example, tissue engineering of a vocal fold replacement is an exciting potential treatment for vocal fold scars that impair vibration and cause voice disturbance. Few reliable options currently exist to improve the dysphonia1, 2, 3, 4. This laboratory has developed a cell-populated three-dimensional tissue-engineered construct intended for vocal fold cover layer replacement5. Human adipose-derived stem cells (ASC) embedded within a clotted cryoprecipitate scaffold differentiate into a bilayered construct intended for vocal fold cover replacement5. This model shows promising function in vitro as a replacement for the vibratory lamina propria and epithelial layers6. An advantage of the design is its composition from all potentially autologous sources. While this model is appealing for human use, it requires adaptation for pre-clinical studies in animals. The vocal folds are exquisitely sensitive to scar formation, and the immune response expected from the xenograft implant of human cells or scaffold may significantly confound their wound healing.
In this work, we set out to develop a tissue-engineered construct suitable for implantation in rabbit vocal folds. Rabbits are a favorable animal model for study of the larynx due to adequate laryngeal size for implantation surgery, histologic similarities to human larynges, and economical animal husbandry relative to larger mammals7. They can undergo phonation both in vivo and in excised larynx preparations8, 9. Furthermore, ASC are easily harvested from rabbits as well as humans. Here we report the production of a tissue-engineered mucosa replacement from rabbit ASC that will allow for pre-clinical construct implantation studies. Key differences in the in vitro development between the human and rabbit cell models are highlighted.
Materials and Methods
Adipose-Derived Stem Cell Harvest and Culture
Rabbit adipose-derived stem cells (rASC) were isolated from rabbit adipose tissue 10. The adipose tissue was acquired post-mortem from animals sacrificed for unrelated studies, in accordance with UCLA Animal Research Guidelines. Inguinal fat pads were harvested in a sterile manner. Tissue was washed in sterile phosphate buffered saline (PBS), minced to increase surface area, and digested in 0.075% type I collagenase (Sigma-Aldrich, St. Louis, MO) in PBS at 37oC for a minimum of 60 minutes with agitation. The solution was then added to “Control Medium” consisting of Dulbeco’s Modified Eagle’s Medium (DMEM), 10% fetal bovine serum (FBS), 1% amphotericin B, and 1% penicillin/streptomycin in a 1:1 ratio to halt digestion and centrifuged at 1600rpm for 5 min. The resulting stromovascular fraction pellet was collected from beneath the fat-laden supernatant, and distributed for culture in control medium on standard tissue culture plates. The cultures were incubated at 37oC with 5% CO2 and medium was changed twice a week until confluent. Confluent cells were passaged or harvested for use on or before the third passage.
Rabbit ASC Differentiation
Rabbit ASC in culture were harvested using trypsin-EDTA and re-seeded in 12-well culture plates. Once confluent, control medium was switched to induce differentiation. Osteogenic differentiation was induced with DMEM, 10% FBS, 0.01 uM 1,25-dihydroxyvitamin D3, 50 uM ascorbate-2-phosphate, 10 mM B-glycerophosphate, and 1% penicillin/streptomycin. Adipogenic differentiation was induced with DMEM, 10% FBS, 0.5 mM isobutyl-metylxanthine (IBMX), 1 µM dexamethasone, 10 µM insulin, 200 µM indomethacin, and 1% penicillin/streptomycin10. All reagents were purchased from Sigma-Aldrich. Both conditions were accompanied by a separate well with control medium as described in cell harvest and culture. Media were changed three times per week for 28 days and then fixed in 4% paraformaldehyde and stained as described in section 2.4.
Fibrin-ASC Constructs
Human cryoprecipitate, rabbit fibrinogen, and bovine fibrinogen were investigated as fibrin sources. Rabbit fibrinogen (Innovative Research) was obtained as a frozen liquid at 5mg/ml in 0.02M Sodium Citrate-HCl at pH 7.4. Bovine fibrinogen (Sigma-Aldrich) was dissolved in 20 mM HEPES-buffered saline at 5 mg/ml. Human cryoprecipitate, pooled from 10 donors, was obtained from the UCLA blood bank in accordance with institutional research policy. All were kept frozen at -80oC until use. Bovine thrombin (Sigma-Aldrich) was prepared at 2U/mL in 20mM HEPES-buffered saline with 7.5mM CaCl2 just prior to its use. The fibrinogen source was mixed in a 4:1:1 ratio with cell suspension and with thrombin solution, in that order, to form fibrin-rASC gels. Initial cell density was 1x106cells/mL to approximate the cell density in true vocal fold lamina propria. The fibrin solution was mixed in a Transwell (Corning) insert for 12-well culture plates, with a porous polycarbonate membrane (0.4µm pore size) at its base. Gelation was achieved within an hour of incubation at 37oC. After gelation was complete, an additional 500,000 rASC were pipetted directly on top of the gel surface to reproduce the cell density of a confluent epithelium. Resultant neotissue constructs were cylindrical, 12mm in diameter, and 1-2mm thick after 2 weeks of incubation at 37oC, 95% humidity and 5% CO2. 1 mL of culture medium was added to the wells and changed 3 times a week until harvest at either 7, 14, or 21 days. 10 ng/mL of human recombinant epidermal growth factor (rhEGF, Promega Corp.) was added to the culture medium of the EGF groups. The Transwell inserts permitted diffusion of the culture medium solely through the porous base membrane.
Histologic Characterization
Tissue constructs were harvested with care to maintain their orientation. Tissue-Tek O.C.T. compound (Sakura Finetek) was used to embed the samples, and the blocks were flash-frozen in liquid nitrogen vapor. Sections were cut at 5 µm on a cryostat, and stored at -20oC until use. For staining, sections were thawed at room temperature and fixed in 4% paraformaldehyde. Hematoxylin and eosin staining was performed on one slide from each construct.
Rabbit larynges were collected within four hours of sacrifice for unrelated experiments, and were frozen at -80oc until use. After thawing, true vocal folds were dissected free from the surrounding cartilage for use as positive controls. Vocal folds were embedded, frozen, cryosectioned, fixed, and stained using the same methods as tissue constructs.
Lipid deposits, indicating adipose differentiation, were identified with Oil Red O staining. Stock solution was prepared using 300 mg Oil Red O (Sigma Aldrich) in 100 mL 99% isopropanol. Sections were rinsed in distilled water followed by 60% ethanol for five minutes each, then stained for five minutes with a working solution comprised of 15 mL Oil Red O stock solution mixed with 10 mL distilled water, incubated at room temperature for 10 minutes. Sections were washed with water and then counterstained with hematoxylin (Richard-Allan Scientific).
Von Kossa solution (5% AgNO3 in distilled water) was used to detect osteogenic differentiation by staining calcium phosphate or calcium carbonate. Sections were rinsed in distilled water for 5 minutes before applying Von Kossa solution and set beneath UV light for ten minutes. Sections were then rinsed in distilled water three times for 5 minutes each and counterstained with hematoxylin for one minute.
All samples were viewed and imaged the same day as staining using light microscopy with an EVOS XL inverted microscope (Life-Technologies).
Immunohistochemistry
For fluorescent immunohistochemistry, constructs were fixed, embedded in paraffin, and sectioned. After de-paraffinization, Trypsin EDTA was added to each section and incubated for 20 minutes at 37oC. Triton X-100 at 0.5% in PBS was applied 3 times for 5 minutes each application. Nonspecific binding was blocked with 10% goat serum (Pierce Biotechnologies) for 60 min and then washed with PBS for 5 minutes. Mouse monoclonal primary antibodies were applied overnight at 4oC in a humidified chamber. Primary antibodies included vimentin (clone AMF17B, Developmental Studies Hybridoma Bank, University of Iowa) at a dilution of 1:100 and prediluted pan-cytokeratin (Abcam ab961). BSA 1% was applied to negative control slides. After washing with PBS 3 times for 5 minutes each, goat anti-mouse secondary antibody tagged with rhodamine was applied for 30 minutes at room temperature at a dilution of 1:400 in PBS. Sections were washed again with 0.5% Triton to remove excess secondary antibody and cover-slipped with VectaShield mounting agent with diamidino-2-phenylindole (DAPI) nuclear stain (Vector Labs, Burlingame, CA).
Fluorescence was viewed and imaged the same day as staining, with an Olympus microscope. Rabbit vocal fold sections were used as positive controls to confirm expected antibody reactivity.
Results
Pluripotency of Rabbit Stromovascular Fraction Cells
Light microscopy demonstrated that cultures induced to differentiate into adipogenic and osteogenic cell types showed morphological changes after 2 weeks in culture (Figure 1). After 28 days in culture, induced cells showed positive staining for lipid after adipogenic culture (Figure 2A) and mineral deposits after osteogenic culture (Figure 2B) while their respective negative controls showed no staining.
Figure 1.Phase contrast microscopy of rabbit ASC cultured under adipogenic (A), osteogenic (B), and control (C) conditions for 14 days on 2-dimensional plastic culture plates. Both experimental conditions have lost the spindle shape and linear arrangement still present in the control. Magnification 20x; scale bar 200m.
Figure 2.Histologic assessment of rASC differentiation after 28 days of culture on 2- dimensional plastic culture plates. Adipogenic culture (A), osteogenic culture (B), and control culture (C and D). Left column shows Oil Red O staining lipid droplets red in (A) but not (C). Right column shows Von Kossa staining mineral deposits black in (B) but not in (D). Magnification 40x; scale bar 100 um; hematoxylin counterstain.
Tissue-Engineered Construct Stability
Rabbit ASC embedded within fibrin gels attached and survived. Constructs formed with rabbit or bovine fibrinogen could withstand handling, manipulation, and placement of 5-0 sutures, irrespective of treatment group or culture period. Constructs formed with human cryoprecipitate degenerated rapidly, could not withstand manipulation past day four, and were not pursued further (Figure 3). Further investigation was performed with the rabbit fibrinogen constructs, for the most relevance for future rabbit implantation.
Figure 3.Rabbit ASC constructs formed with human cryoprecipitate show progressive vacuolization and compaction on days 1(A), 4 (B), and 7 (C). Magnification 4x; scale bar 1 mm.
For the constructs made with rabbit fibrinogen, hematoxylin and eosin microscopy showed a fibrin lattice with similar gross morphology to the vocal fold lamina propria and epithelium. Cell nuclei were identified throughout the construct (Figure 4). There was no observable morphologic difference between constructs treated with EGF and those that were not.
Figure 4.Hematoxylin and eosin staining of rabbit true vocal fold (A) and a fibrin-rASC construct (B). The construct after 14 days of culture with EGF demonstrates a bilayered structure with cells attached throughout the construct. Magnification 40x; scale bar 100 µm.
Differentiation of Rabbit ASC in Fibrin Gel Culture
Spontaneous differentiation to mesenchymal phenotypes did not occur. Histological staining using Oil Red O and Von Kossa on tissue-engineered constructs in cultured for 14 days, with and without EGF treatment, showed negative staining for lipid and mineral (Figure 5).
Figure 5.Histologic assessment of rASC differentiation in fibrin constructs after 14 days in culture without EGF (top row) and with EGF (bottom row). Von Kossa (A and C) and Oil Red O (B and D) show no staining respectively for either mineral or lipid. Magnification 20x; scale bar 200 µm, counter-stain with hematoxylin in all.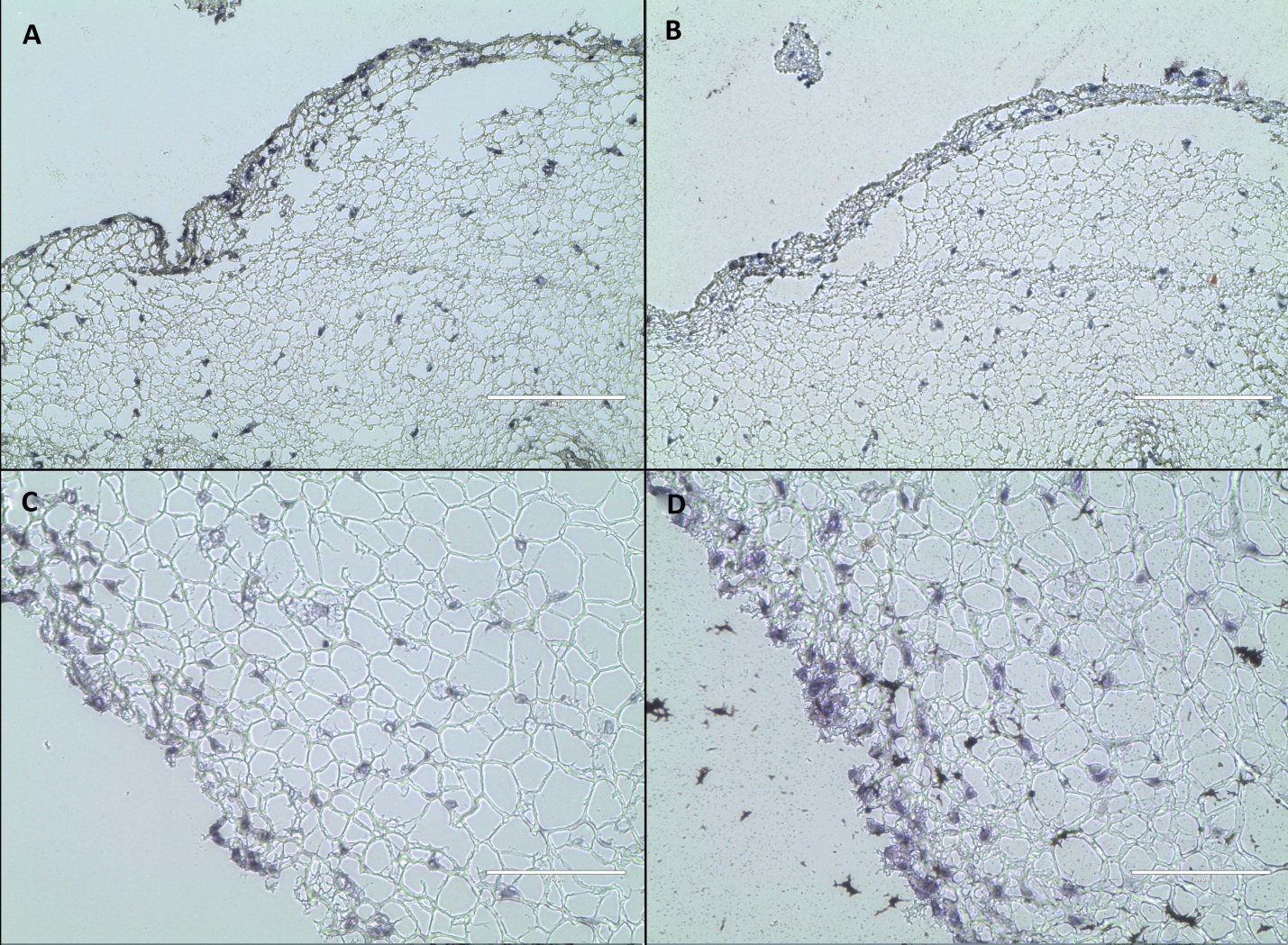
Epithelial differentiation was not induced by EGF and an air interface in this rabbit ASC culture, as indicated by immunohistochemistry. Immunohistochemistry at 14 days was negative for cytokeratin. Vimentin, a general mesenchymal cell marker, was preserved in all cells with and without EGF exposure (Figure 6).
Figure 6.Immunohistochemistry in rabbit true vocal fold (left column), and a fibrin-rASC construct after 14 days in culture with EGF (right column). Vimentin (top row) and cytokeratin (bottom row) appear red, DAPI-labeled nuclei appear blue. A. Vimentin is expressed throughout vocal fold lamina propria. B. Vimentin is expressed in all cells within the construct. C. Cytokeratin is expressed in all cells in the vocal fold epithelium. D. Cytokeratin is indistinguishable from negative control construct samples. Magnification 40x.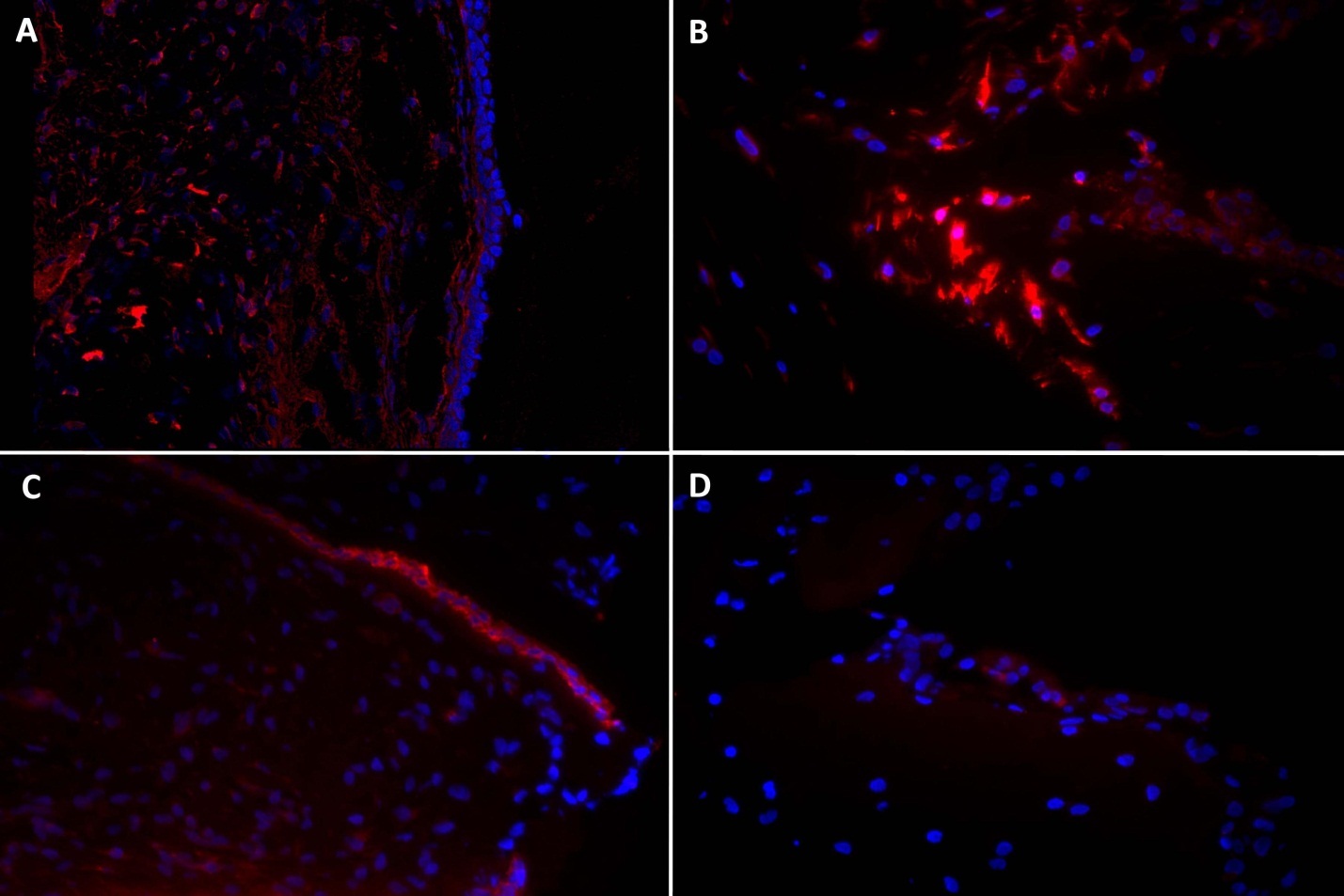
Discussion
Tissue engineering and regenerative medicine hold promise for diseases that are currently difficult to treat because of lack of suitable therapies or self-repair mechanisms. Vocal fold scarring is an excellent example. The specialized extracellular matrix of the vocal fold lamina propria and its attached epithelium are, thus far, irreplaceable after injury. Its geometry also makes the vocal fold mucosa a good candidate for tissue engineering in the laboratory. It is a small, thin structure with low metabolic activity due to sparse cellularity; these factors ensure adequate nutritional perfusion without need for neovascularization.
Befitting the label “stem cells”, adipose-derived stem cells possess the capabilities of self-renewal and multiple differentiation potential11. ASC may be more clinically applicable than other stem cell types because they are quite easily harvested from adult liposuction material and are simple to maintain in culture12. As such, ASC have been proposed as a cell source for engineering and repair of nearly any conceivable tissue type13, 14. They are currently being investigated in nearly one hundred active human clinical trials, primarily of cell injections alone without scaffolds15. Of particular interest are the immune modulating and wound healing properties of these cells16 Development of more complex three-dimensional tissue and organ replacement is still in the in vitro and pre-clinical animal implantation phases.
We focused our attention here on rabbit ASC, since rabbits are an ideal small animal model for studies of the larynx. The larynx is accessible, adequately sized, able to phonate in an excised setting; and histologically and structurally similar to the human larynx. Furthermore, the inherently silent behavior of rabbits minimizes disruption to the healing implant. Multi-potent rabbit ASC are capable of differentiating into bone and fat-like lineages, as demonstrated here and in prior studies17. Rabbit ASC have been shown to differentiate into an epithelial phenotype when exposed to an air liquid interface and a growth factor cocktail, consisting of all-trans retinoic acid, EGF, and further enhanced by hepatocyte growth factor and keratinocyte growth factor for 12 days18. That study was not performed in a three-dimensional scaffold.
Fibrin was chosen as the biological scaffold for this study due to its importance in normal wound healing, its biodegradable nature, and its potential for autologous sourcing from plasma. As the principal proteinaceous component of blood clots, fibrin is formed when the enzyme thrombin cleaves end-terminal peptides from circulating fibrinogen protein. Exposing the fibrinogen terminals allows rapid self-polymerization, and long fibrin chains result. Initial attempts at embedding the rabbit ASC used human cryoprecipitate as fibrinogen source, as was previously used for human ASC5. However, unlike human ASC, the rabbit ASC rapidly degraded and compacted the cryoprecitate-based fibrin; constructs were largely degenerated within seven days. Thus a commercial purified fibrinogen isolated from rabbit plasma was utilized, and this created durable constructs that were handled beyond 21 days without difficulty or breakdown. Potential explanations for this difference are hypothesized. Cryoprecipitate is an unpurified source of clotting factors from plasma, which does include other bound proteins such as matrix metalloproteinases that could degrade the scaffold. It is also possible that human antigens from the cryoprecipitate activated the rabbit ASC to secrete degradatory enzymes. With either hypothesis, the net balance of proteinases to inhibitors likely differed between the human and rabbit cell cultures. Also, fibrinogen concentration within the cryoprecipitate was unknown and likely differed from that used in the pure form. Finally, some of the volume loss appeared due to greater cell-mediated contraction in the cryoprecipitate case, either due to stronger cell adhesion or a more contractile state. Regardless of the cause, the excess scaffold loss with human cryoprecipitate highlights the importance of designing each component of an implant for the intended species and target tissue. The less contractile case with rabbit fibrinogen is better suited to implant in the vocal fold where preventing repeat scar formation is paramount. Also, avoiding human antigens in the rabbit implant will reduce the risk of immune rejection. Similarly, rabbit fibrinogen was preferred over the more readily available bovine fibrinogen, to avoid undue antigen exposure during ultimate implantation in rabbit vocal folds.
For eventual human implantation, a fibrin scaffold could be produced from either autologous cryoprecipitate or pooled human donor cryoprecipitate. The risk of viral transmission or inflammatory reaction from allogeneic human cryoprecipitate implantation in the solid form is not well delineated, but is likely to be low since human pooled fibrin and thrombin-based products have been safely used for surgical hemostasis for many years19. Interestingly, even the use of bovine aprotinin components only very rarely leads to a clinical immunologic response in humans20. Regardless, a clear advantage of fibrin as a scaffold is that it can be prepared from human plasma for autologous re-implantation without risk. This would be feasible in rabbit implant studies as well, but would significantly increase the complexity of an experimental series by requiring pre-implantation plasma collection and animal-specific culture.
We did investigate epithelial differentiation in this system, but found that all cells primarily maintained a mesenchymal phenotype. Unwanted mesenchymal differentiation to produce lipid or mineral deposits did not occur. Li et al demonstrated differentiation of rabbit ASC to epithelial-like cells in a monolayer air liquid interface system, with nearly 70% epithelial-like differentiation17. They used a thin collagen type IV coated membrane with a variety of growth factors to induce high levels of cytokeratin 19 (an early epithelial marker) staining and low levels of cytokeratin 13 (a mucosal epithelial marker). In our system using EGF treatment in a fibrin gel, as was previously used with human ASC, clear expression of the epithelial marker CK19 was not evident after two weeks in culture. This difference between human and rabbit cell behavior again highlights the importance of species-specific investigation.
The construct developed here, even without significant epithelial differentiation, is suitable for implantation as a vocal fold mucosa graft in rabbits. The in vivo microenvironment may direct differentiation of the superficial layer of cells to an epithelial phenotype. ASC or bone marrow derived stem cell injections into animal vocal folds in vivo decrease scaring and dense collagen deposition; improve viscoelasticity and mobility; and increase elastin production, vocal fold smoothness and collagen organization9, 21, 22, 23, 24. Furthermore, adding a micronized acellular dermal matrix or hydrogel scaffold to the injection decreased scarring9, 25. The wound healing properties of ASC may be adequate to prevent scar formation after implantation, while the fibrin scaffold may further support ASC survival and differentiation. These aspects will be tested in a rabbit implantation trial.
Conclusions
Rabbit adipose-derived stem cells were used to create a three-dimensional tissue-engineered construct suitable for vocal fold cover replacement. The construct resembled the vocal fold lamina propria and epithelium in microstructure although not in cell phenotype. The construct was able to withstand handling and suturing similar to a native rabbit vocal fold cover layer. Notable differences were found in cell differentiation and scaffold degradation between human and rabbit ASC. This construct will be used for future in vivo implantation studies in rabbits.
Acknowledgements
Vimentin antibody was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by the University of Iowa, Department of Biology, Iowa City, IA 52242.
References
- 1.Welham N V, Choi S H, Dailey S H, Ford C N, Jiang J J. (2011) Prospective multi-arm evaluation of surgical treatments for vocal fold scar and pathologic sulcus vocalis. , Laryngoscope(2011); 121, 1252-60.
- 2.Chhetri D, Berke G.Injection of cultured autologous fibroblasts for human vocal fold scars. (2011)a Laryngoscope. 121, 785-92.
- 3.Thibeault S L, Klemuk S, Smith M E, Leugers C, Prestwich G.In vivo comparison of biomimetic approaches for tissue regeneration of the scarred vocal fold (2009) Tissue Eng: Part A;. 15, 1481-7.
- 4.Johnson B Q, Fox R, Chen X, Thibeault S. (2010) Tissue regeneration of the vocal fold using bone marrow mesenchymal stem cells and synthetic extracellular matrix injection in rats. , Laryngoscope; 120, 537-55.
- 5.Long J, Zuk P, Berke G, Chhetri D. (2010) Epithelial differentiation of adipose-derived stem cells for laryngeal tissue engineering. , Laryngoscope; 120, 125-31.
- 6.Long J, Neubauer J, Zhang Z, Zuk P, Berke G et al. (2010) Functional testing of a tissue-engineered vocal fold cover replacement. , Otolaryngology-Head and Neck Surgery 142(3), 438-440.
- 7.Bless D M, Welham N V.Characterization of vocal fold scar formation, prophylaxis, and treatment using animal models (2010). Curr Opin Otolaryngol Head Neck Surg;18:. 481-486.
- 8.Ge P, French L, Ohno T, Zealear D, Rousseau B.Model of evoked rabbit phonation (2009). Ann Otol Rhinol Laryngol;118:. 51-5.
- 9.Maytag A L, Robitaille M J, Rieves A L, Madsen J, Smith B L. (2013) Use of the rabbit larynx in an excised larynx setup. , J Voice; 27, 24-28.
- 10.Zuk P A, Zhu M, Ashjian P, De Ugarte DA, Huang J I. (2002) Human Adipose Tissue is a Source of Multipotent Stem Cells. , Molecular Biology of the Cell; 13, 4279-4285.
- 11.De Ugarte DA, Morizono K, Elbarbary A, Alfonso Z, Zuk P.Comparison of multi-lineage cells from human adipose tissue and bone marrow (2003). Cells Tissues Organs;. 174, 101-9.
- 12.Zuk P A, Zhu M, Mizuno H, Huang J, Futrell J W. (2001) Multilineage cells from human adipose tissue: implications for cell based therapies. , Tissue Engineering; 7, 211-228.
- 14.Baer P C. (2014) Adipose-derived mesenchymal stromal/stem cells: An update on their phenotype in vivo and in vitro. , World J Stem Cells; 6(3), 256-65.
- 16.Leto Barone AA, Khalifian S, WPA Lee, Brandacher G Doi. (2013) 10.1155/2013/383685. Immunomodulatory Effects of Adipose-Derived Stem Cells: Fact or Fiction? BioMed Research International.(2013):. 383685.
- 17.Li H, Xu Y, Fu Q, Li C.Effects of multiple agents on epithelial differentiation of rabbit adipose-derived stem cells in 3D culture (2012). Tissue Eng Part A;18:. 1760-70.
- 18.Sa Barretto LS, Lessio C, ANS Nakamura.Cell kinetics, DNA integrity, differentiation, and lipid fingerprinting analysis of rabbit adipose-derived stem cells. In Vitro Cellular & Developmental Biology – Animal. October2014; 50(9), 831-9.
- 19.Spotnitz W D, Mintz P D, Avery N, Bithell T C, Kaul S. (1987) Fibrin glue from stored human plasma. An inexpensive and efficient method for local blood bank preparation. , Am J Surg 53, 460-462.
- 20.Siedentop K H, Park J J, Shah A N, Bhattacharyya T K, O’Grady K M.Safety and efficacy of currently available fibrin tissue adhesives. , Am J Otolaryngol; 22, 230-235.
- 21.Greenhalgh D G, Gamelli R L, Lee M, Delavari M, Lynch J B.Multicenter trial to evaluate the safety and potential efficacy of pooled human fibrin sealant for the treatment of burn wounds. , J Trauma(1999); 46, 433-440.
- 22.Beierlein W, Scheule A M, Antoniadis G, Schosser R.An immediate, allergic skin reaction to aprotinin after reexposure to fibrin sealant. , Transfusion(2000); 40, 302-305.
- 23.GCB Medrado, Machado C B, Valerio P, Sanches M D, Goes A M.The effect of a chitosan-gelatin matrix and dexamethasone on the behavior of rabbit mesenchymal stem cells (2006). Biomed Mater;1:. 155-61.
Cited by (1)
- 1.Goel Alexander N., Gowda Bhavani S., Veena Mysore S., Shiba Travis L., Long Jennifer L., 2018, Adipose-Derived Mesenchymal Stromal Cells Persist in Tissue-Engineered Vocal Fold Replacement in Rabbits, Annals of Otology, Rhinology & Laryngology, 127(12), 962, 10.1177/0003489418806008
