A Study on the Feasibility and Utility of Continuous Glucose Monitors in Elite Football
Abstract
Physiological performance may be limited by reduced systemic glucose availability to working muscles. Continuous glucose monitors (CGM) measure interstitial glucose every 1-15 minutes, offering a practical way to assess glucose during sporting activity. However, empirical research has predominantly focused on endurance-based sports, whereas glucose responses during professional competitive football matches remain unknown. This study evaluated the feasibility and utility of CGM in professional football. Eight professional, male outfield footballers from the English third tier participated in the study. Participants completed a 14-day food diary control period, followed by a 28-day observation period wearing CGM devices during six matches and sixteen training sessions. CGM devices remained in situ for 98% of training sessions and matches. Mean glucose concentrations were 6.5 ± 1.2 mmol/L during training sessions, 7.5 ± 2.1 mmol/L during match play, and 5.4 ± 0.3 mmol/L overnight. No significant differences were found between glucose concentrations during match play (p = 0.060) or training (p = 0.510), compared to overnight fasted glucose concentrations. There was also no difference between training and match-play glucose concentrations (p = 0.788). Glucose concentrations were highly individualised, with one player displaying minimal change throughout match play (-0.2 mmol/L) whereas another experienced increases of up to 5.8 mmol/L. Non-nutritional factors appeared to influence glucose concentrations; participants (n=3) who used nicotine pouches displayed an transient increase in blood glucose in the 10-55 minutes after administration. This study concludes that CGM use in professional football is feasible for assessing individual glucose responses to training and match-play.
Author Contributions
Copyright © 2025 Sophie Harries, et al
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have no conflict of interest to declare.
Citation:
Introduction
Intermittent team sports and endurance sports rely on glycogenolysis to maintain blood glucose concentrations 1. Sustained periods of muscle glycogenolysis lead to significant muscle glycogen depletion 2, progressively reducing energy substrate availability which may contribute to the development of peripheral muscle fatigue 3 and a subsequent reduction in repeated sprint activity and match performance 4. Research by Krustrup et al. 3 empirically documents these metabolic changes, demonstrating that muscle glycogen levels can decrease by 40-60% during a typical match, depending on playing intensity and individual metabolic characteristics. Reduced glucose availability, as a result of either low blood glucose, low glycogen stores or low carbohydrate intake, is also associated with the development of central fatigue 1 due to reduced brain glucose uptake 5. Although fatigue is multi-factorial preserving blood glucose concentration can reduce the reliance on glycogen for carbohydrate oxidation, and may therefore delay an aspect of fatigue during match-play, benefitting tactical and physical performance 6. Although the relationship between blood glucose concentrations and muscle glycogen is not fully understood 7, maintaining blood glucose increases carbohydrate availability, and helps to meet the metabolic demand of exercise 8. However, the optimal blood glucose concentration for exercise performance is not known and therefore the monitoring of glucose concentrations in a sporting population may be of value.
Traditionally, blood glucose concentrations have been measured using fingertip capillary blood sampling. In most contexts, this is impractical, causes discomfort, and only provides discrete data on a measure that changes continually during exercise 9. Continuous Glucose Monitors (CGM) are now routinely used within healthcare services to provide a continuous measurement of interstitial glucose concentrations. This has revolutionised healthcare for people with diabetes 10, with real-time interstitial glucose data helping people to monitor glucose concentrations, and patients to avoid hyperglycaemia (>10 mmol/L) or hypoglycaemia (<3.9 mmol/L) without medical intervention 11. In the context of sport, the ability to continuously monitor glucose concentrations pre, during and post-exercise may help to inform fuelling strategies related to carbohydrate type and timing of ingestions with the aim of optimising performance and recovery 12. However, the benefits of using CGM in a sporting setting is far from established.
Research using CGM in professional sport is emerging. Previous studies evaluating CGM have focussed on sports where a need for high carbohydrate availability is most well established in relation to performance, such as endurance cycling 13 and running 14. Studies employing CGM have reported high levels of individuality in glucose concentrations, beyond that of measurement error, in response to both exercise and carbohydrate intake even with a standardised diet 13, 14, 15. Individuality in response could be influenced by stress, illness, dehydration or hormones 16, therefore identifying individualised dietary or exercise responses can be hindered by non-dietary factors that influence glycaemic control. Carbohydrate absorption, metabolism, uptake and utilisation 17, eating behaviour 19, and commonly reported differences in gut microbiome 18, could also be impacted by genetic variation. Therefore, it could be argued that biometric feedback could be used for athletes to tailor a nutrition plan that accounts for individual differences 20. Previous studies have reported limited adverse effects of CGM use, and the data from CGM have been used to inform athlete fuelling and race pacing strategies 13, 14. Based on these findings, CGM may be used within endurance sports to allow for bespoke fuelling strategies that help to mitigate fatigue and improve athletic performance. Of note, the use of CGM during competition has recently been banned in professional cycling due to concerns around personal privacy, financial accessibility, and the influence of technology on human racing 21. While these factors should be carefully considered by governing bodies, the existing research demonstrates the potential for biometric information to positively inform bespoke fuelling strategies within endurance sport. Other sports that involve an endurance component, such as team sports, could similarly benefit from CGM. However, there is limited research assessing their practical use. For example, the research conducted in football is restricted to simulated match-play of semi-professional players using a standardised treadmill protocol 22. However, this limits the extent to which the data collected can be generalised to a competitive match because these methods do not account for the contact nature of competitive football (which could dislodge sensors), and within-player variability in metabolic demand due to the variable dynamics of professional match-play 23.
Therefore, the aim of the present study was to evaluate the feasibility and utility of CGM use within professional footballers. Feasibility was defined as retention of CGM during an in-season competitive period. Utility was defined as the potential for the biometric data collected to impact nutrition strategies. A secondary aim was to determine the interstitial glucose concentrations during training and match play.
Methods
Participants
Eight professional male (age: 28.0 ± 5.5 years, body mass:86.4 ± 4.8 kg, height: 1.8 ± 0.1 m) outfield footballers competing in the third tier of English football during the 2023/24 season volunteered for the study. Participants had no history of diabetes and provided written informed consent. Ethical approval was granted by Nottingham Trent University Ethics Committee.
Study Design and Procedures
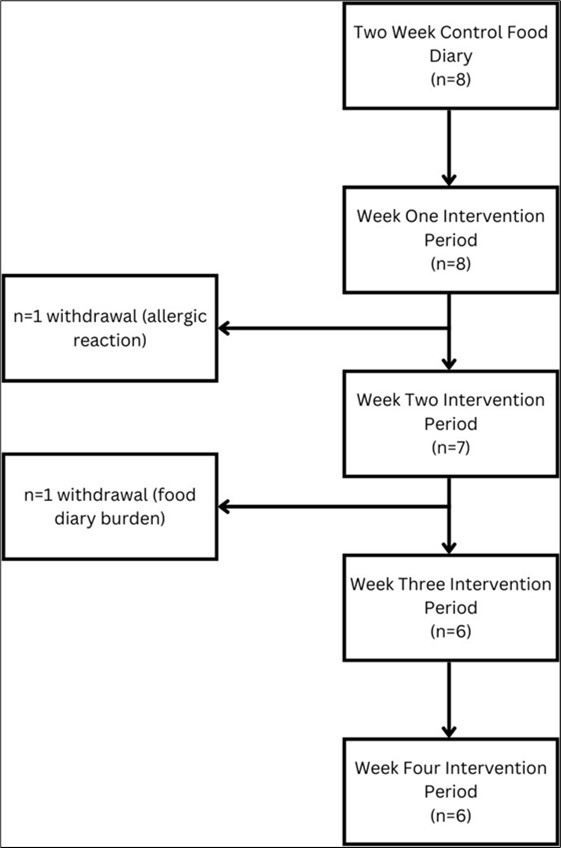
Participants completed a 14-day control (112 total player days) period, during which they reported dietary intake. This was followed by a 28-day observation period (188 total player days), whereby participants wore the CGM (Abbot Libre Sense Glucose Sport Biosensor, United States) and continued to report dietary intake. Two participants withdrew from the study during the observation period (Figure 1).
Continuous Glucose Monitors
CGM are reported to have a lag time of 1.2 - 7.0 minutes and >90% of sensor readings are within 20% of blood glucose concentrations 24. The device has an upper limit of 11.2 mmol/L. The device was inserted into the back of the upper arm by the researcher and replaced after 14 days, as per manufacturer instructions 25. Specialist manufacturer-supplied performance patches, made of a silicone protection ring and 4-way stretch kinesiology tape, were placed onto the device for protection. The CGM was paired with the user’s smartphone application 26 via Bluetooth for minute-by-minute data collection. If the device was disconnected from Bluetooth (e.g., due to distance between participants and their smartphone), readings were taken every 15 minutes and stored by the device for 8 hours until it was re-synchronised via Bluetooth. Post-training, at half-time and post-match, participants were instructed to re-synchronise the CGM device with their smartphone via Bluetooth.
Dietary logs
Participants reported dietary intake using a Snap and Send method, aimed to reduce reporting bias 27. Participants took photos of all food and drink consumed alongside an object of known size (massage ball, 8cm diameter) at 45- and 90- degree angles. Participants received individual demonstrations regarding the dietary reporting method with common troubleshooting issues discussed, e.g., complex dishes with multiple ingredients to be listed alongside the photograph. Images were sent via an instant messenger service (Whatsapp, Meta Platforms Inc.) to the primary researcher. If participants had not sent a photo through within 2 hours of their usual predicted mealtime, the researcher reminded participants via a text message e.g., breakfast reminder was sent at 10:30 in anticipation that a meal would have been consumed around 08:30. If participants failed to photograph their meal, dietary recall information was provided with portion sizes estimated. The primary researcher was present when meals were consumed at the training centre and assisted with dietary reporting by photographing and documenting supplement use. Participants reported all supplement use as part of their food diary, examples include caffeine gum, beta-alanine, creatine, and electrolytes. If supplements were provided for the participants by the club, dosage was confirmed with the team’s nutritionist. For supplements taken at home, participants reported brand, weighed measure or dosage. On Day 2 of the observation period, the potential impact of tobacco free nicotine pouches, alongside tobacco containing Snus, on glucose concentration was observed and, therefore, nicotine pouch/Snus use was reported on Day 2 onwards. Nicotine pouch/Snus administration times and corresponding CGM value were time aligned based on the known delay (2.4 ± 4.6 minutes) with CGM readings 24.
Training Load
During the observation period, three home matches and three away matches were played. Training schedules (16 sessions) and match day squad selection were not adjusted or influenced based on CGM study involvement or findings. Global Positioning System (GPS) units (Vector, Catapult, Australia) were positioned between the shoulder blades using a manufacturer-supplied vest and worn throughout training and match-play. Post-training and match-play, each GPS data set was downloaded and analysed using commercially available software (Viper, STATSports, Ireland). The physical performance variables assessed included: total distance covered (m), high speed (>5.5 m/s) distance covered (m), sprint speed (>7.0 m/s) distance covered (m), number of accelerations above 0.5 m/s2 for >0.5 s, and number of decelerations below −0.5 m/s2 for >0.5 s.
Feasibility
The feasibility of CGM was assessed based on the percentage of completed training and match sessions during which the device remained in situ with data available for analysis. Additionally, any adverse effects reported by participants, particularly discomfort, were recorded.
Statistics
Overnight fasted glucose concentrations were determined by calculating the mean of overnight glucose concentrations from 00:00-06:00 during Week 1 of the observation period. This mean was used as a baseline measure. Glucose concentrations were assessed three hours pre-match, first half, half-time, second half, three hours post-match, and for training sessions, for each participant. Mean concentrations are reported during each period. During training and match play, the Bluetooth connection to CGM was lost, due to the distance between the monitor and the player’s smartphone, a singular 5-minute mean glucose concentrations were recorded. As such, first and second half match play was restricted to three measurements in 15-minute intervals. Percentage and absolute change from overnight fasted interstitial glucose concentrations were calculated for all match-play and training session measurements.
A Shaprio-Wilk’s test was conducted to assess normality in distribution of data with all data confirmed as normally disturbed. Paired T-tests, with a significance level of p < 0.05, and Bonferroni correction were used to assess the difference between mean glucose concentrations during training sessions and match play (inclusive of first half, half time and second half), overnight fasted glucose concentrations and training, and overnight fasted glucose concentrations and match-play. A paired T-test was also used to assess differences in dietary intake between the control and observation periods.
Dietary intake was analysed by a Sports and Exercise Nutrition registered (SENr) dietitian, using dietary analysis software (Nutritics, Version 5). Dietary intake was quantified as energy (kilocalories), carbohydrates (grams) and protein (grams) in absolute units and relative to body mass. Mean energy and macronutrient intake between the control and observation period were compared to establish any significant changes for the duration of the study using a paired T-test, following normality testing (Nutritics, Version 5).
Results
Feasibility
CGM were used in 148 training or match events, and needle displacement occurred on three occasions, all during training, thus the CGM produced data for 98% of training and match play events without displacement. Six participants regularly participated in training and match play. Four of the six participants sustained short-term muscular strain injuries, which resulted in a total of 9 player days of modified training i.e ‘off feet’ indoor gym work. Two participants were injured prior to the study start date and did not return to training or match play during the study period, participating only in rehabilitation sessions. However, these participants continued to wear the CGM and record dietary intake throughout their rehabilitation.
Dietary Intake
Carbohydrate intake did not differ between the control and observation periods (control 3.0 ± 0.6 g/kg/day, observation period 3.2 ± 0.7 g/kg/day) (t = 1.595, p = 0.077). There was no difference in energy and protein intake between the control and observation periods (t = 0.028, p = 0.489 and t = 0.016, p = 0.494, respectively). Dietary intake values can be seen in Table 1. Dietary recall rather than the Snap and Send method was used for one participant two days preceding drop out.
Table 1. Dietary intake values for energy, carbohydrate and protein across the study duration (Overall), Match Day-1, Match Day and Match Day+1. Values are mean ± SD and expressed in absolute and relative units (n=8)| Overall | Match Day -1 | Match Day | Match Day +1 | |
| Energy (kcal/day) | 2704 ± 410 | 3377 ± 644 | 2808 ± 759 | 2024 ± 387 |
| Energy (kcal/kg BM) | 31.3 ± 4.7 | 39.0 ± 6.6 | 32.7 ± 9.5 | 23.5 ± 4.9 |
| Carbohydrates (g/day) | 270 ± 53 | 369 ± 96 | 317 ± 71 | 176 ± 38 |
| Carbohydrates (g/kg BM) | 3.1 ± 0.6 | 4.2 ± 0.9 | 4.2 ± 0.9 | 2.1 ± 0.6 |
| Protein (g/day) | 161 ± 34 | 201 ± 46 | 146 ± 6 | 115 ± 27 |
| Protein (g/kg BM) | 1.9 ± 0.4 | 2.3 ± 0.5 | 1.7 ± 0.7 | 1.3 ± 0.3 |
Response to Training and Match Play
The demands of training (mean total distance = 3930 ± 432 m, mean accelerations = 59 ± 13, mean decelerations = 47 ± 10, mean high-speed running distance = 143 ± 94 m) and match-play (mean total distance = 6044 ± 3700 m, mean accelerations = 84 ± 35, mean decelerations = 82 ± 33, mean high-speed running = 294 ± 256 m) were captured during all sessions.
Mean glucose concentrations were 6.5 ± 1.2 mmol/L during training sessions, 7.5 ± 2.1 mmol/L during match play, and 5.4 ± 0.3 mmol/L overnight. There were no significant differences in glucose concentrations in response to match-play (p = 0.060) or training (p = 0.510), compared overnight fasted interstitial glucose concentrations. There was also no significant difference between training and match-play glucose concentrations (p = 0.788) (Figure 2).
Figure 2.Box and Scatter plot with individual player means for blood glucose 3 hours Pre- match, 1st half match play, half-time, 2nd half match play and 3 hours post-match.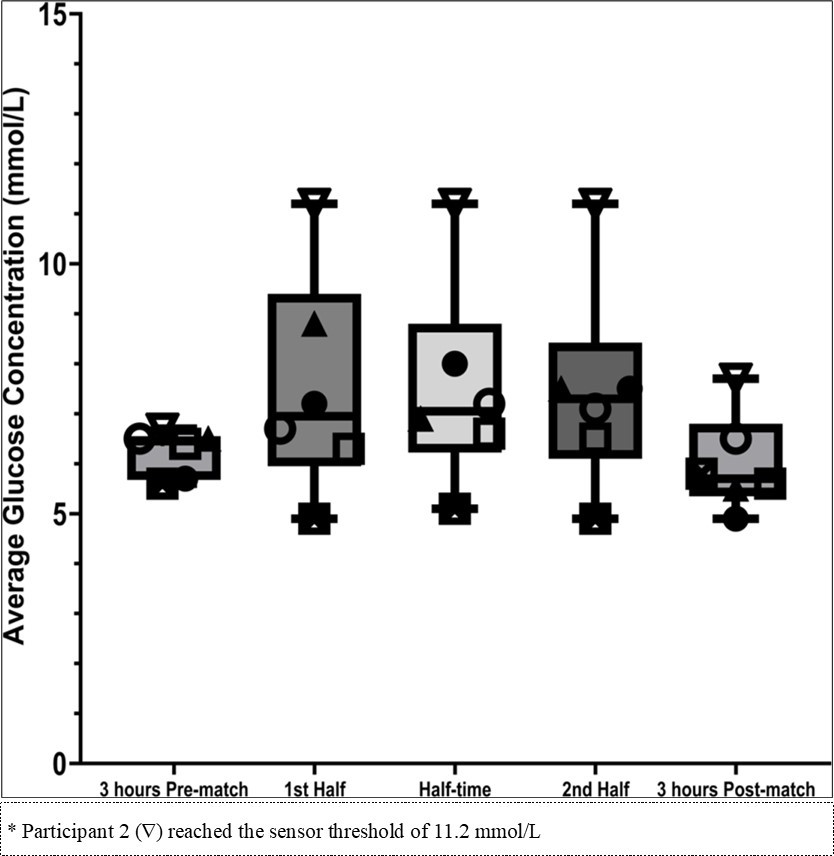
Inter-Participant Variability
Glucose concentrations during match-play varied between participants (Figure 2 and Table 2). During the first half of match-play the absolute change in glucose concentrations from overnight fasted glucose concentrations was between - 0.2 mmol/L and + 5.8 mmol/L and similar changes were seen during the second half of match play ( - 0.2 mmol/L and + 5.8 mmol/L).
Table 2. Absolute and Percentage change in mean glucose concentrations during match-play compared to mean overnight fasted glucose concentrations.| Player ID | 1st half absolute change (mmol/L) | 1st half percentage change | Half time absolute change (mmol/L) | Half-time percentage change | 2nd half absolute change (mmol/L) | 2nd half percentage change |
| 1 | 2.2 | 42.4 | 2.9 | 56.8 | 2.4 | 47.1 |
| 2 | 5.8 | 106.8 | 5.8 | 106.8 | 5.8 | 106.8 |
| 3 | 3.0 | 50.1 | 1.0 | 17.7 | 1.6 | 26.6 |
| 4 | 1.3 | 23.1 | 1.8 | 32.5 | 1.7 | 30.4 |
| 5 | 0.5 | 9.1 | 0.8 | 13.9 | 0.7 | 11.6 |
| 6 | -0.2 | -4.2 | 0.0 | 0.1 | -0.2 | -3.7 |
Effects of nicotine pouch use on glucose concentration
Nicotine pouch or Snus administration (n=3) resulted in a peak increase (mean for the 3 individuals) in glucose concentration after nicotine pouch/Snus was 4.1 mmol/L. These three examples of the interstitial glucose response to nicotine pouches or Snus are demonstrated in Figure 3. These changes occurred in the absence of dietary intake and/or physical activity at the time of administration.
Figure 3.Glucose concentration before and after nicotine pouch or Snus administration (0 minutes) without the presence of diet or exercise factors.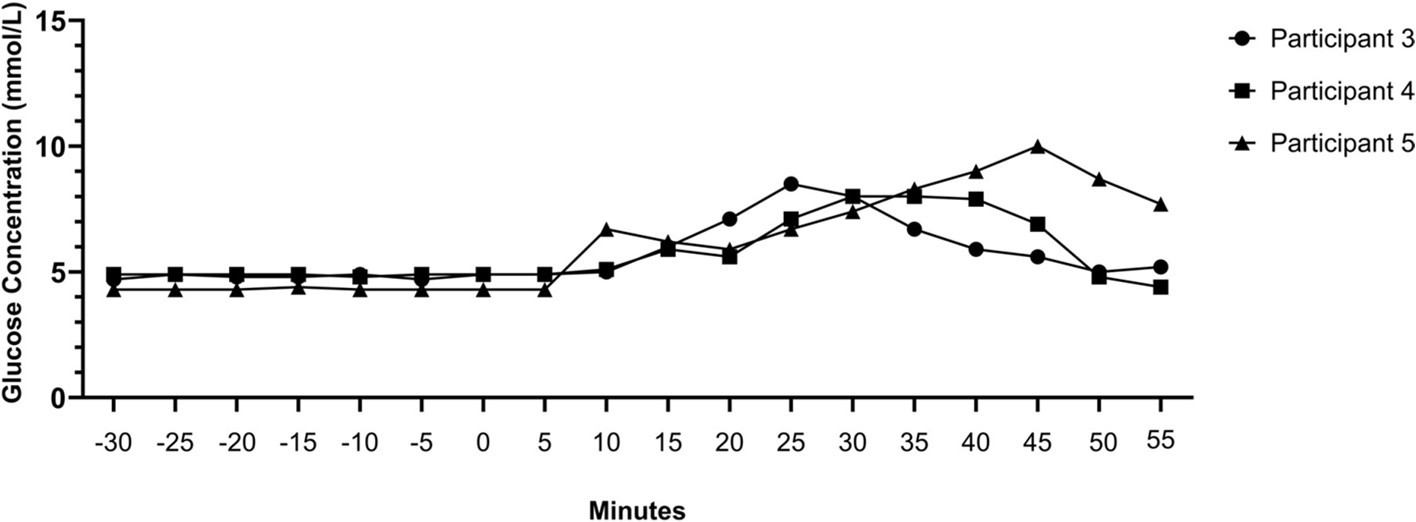
Discussion
Feasibility
The primary aim of this study was to assess if the use of CGM technology was feasible and useful within professional football. This study demonstrated that CGM can be feasibly used to monitor glucose concentrations in professional football, evidenced by successful recording in 98% of the training or match play events (135 hours and 57 minutes of match play and on-pitch training sessions). Previous studies have reported limited adverse effects of CGM use, and the biometric data from CGM has been used to inform athlete fuelling and race pacing strategies 13, 14. Based on these findings, CGM has the potential to be used within sports to allow for bespoke fuelling strategies that could help mitigate fatigue and improve athletic performance, through immediate insights into an athlete’s physiological energy utilisation and response. The ban on CGM use in professional cycling 21 due to the technology's influence on racing seemingly supports their benefit 31. CGM also revealed some individual variability in glucose concentrations during match play and some unexpected changes in glucose concentration in response to nicotine pouch/Snus administration. In summary, these findings suggest there may be value of using CGM in professional footballers, to better understand the individual responses to nutrition and lifestyle, and identify players who may benefit from personalised support strategies.
Response to Match Play
CGM identified individual differences in blood glucose response to training and match-play. The difference between the highest and lowest glucose concentration was 5.9 mmol/L. The metabolic demands of football vary from match to match and between players 28 and this may partially contribute to the individuality observed in the present study. However, individuality has also been reported in endurance athletes 13, 14, where metabolic demands may be more consistent. As observed by Bowler et al., 28, day-to-day variability between athletes was more prominent than overnight variability, and secondary to diet and activity induced changes in glucose concentrations. Variability between individuals is likely to be associated with genetic differences impacting carbohydrate absorption, metabolism, uptake, and utilisation 17. Based on the variability observed in the present and previous studies, monitoring individual glucose responses using a CGM device could allow practitioners to develop personal fuelling strategies that account for individual variation in glucose availability during exercise 30, 31. Despite this, there is no existing data on ‘optimal’ blood glucose range for performance in football, so the value of monitoring and/or responding to glucose changes is debatable. Hiromatsu et al., (2023) concluded that it is likely to be important to maintain high glucose availability during match-play, to ensure provision of a continuous source of glucose to fuel carbohydrate metabolism, required for high-intensity efforts. However, the value of monitoring glucose concentrations via CGM for performance in football remains to be established.
CGM has improved care for people living with diabetes, with multiple studies demonstrating improvements in glycaemic control and reduced reliance on glucose-lowering medication in patients using CGM 10. Much of this success is attributed to the educational value these monitors provide patients, encouraging changes in diet and physical activity to improve glucose concentrations, leading to improved health outcomes 32. Whether continuous monitoring of glucose concentrations via CGM can influence behaviour in athletes in currently unknown. Many athletes struggle to achieve the carbohydrate intake recommendations 34, with our study finding that players did not reach the UEFA recommended carbohydrate intake of 6-8 g/kg BM 34. Interestingly we did observe a non-statistically significant tendency (p = 0.077) for carbohydrate intake to increase slightly during the CGM observation period. Although the absolute difference is small (0.2 g/kg BM), there are known limitations of food diaries to accurately capture intake 27, and as such, this finding could be interpreted as indicative of change rather than an accurate reflection of absolute intake. Further research is required in a larger sample size and over a longer time frame to better understand the potential educational value of CGM in athletes to positively influence dietary intake behaviours.
One significant consideration for practitioners surrounding the utility of CGM is that during match-play CGMs are not synchronised. This results in an mean reading being recorded every 15 minutes rather than minute by minute. There is also an approximate 2.4 ± 4.6 minutes minute delay before interstitial glucose concentrations reflect blood glucose concentrations 24, which needs to be accounted for by practitioners. However, with sufficient duration and repetition, there may be potential for CGM to generate a ‘blood glucose profile’ for individual players, which could allow for proactive nutritional approaches.
Other Insights
An advantage of CGM is the capturing of unexpected effects of diet or behaviour on glucose concentrations. In the present study, three of the eight participants regularly used either a tobacco free nicotine pouch or tobacco containing Snus pouch, administered between the gum and upper lip. Nicotine pouches and Snus are reported to help relaxation and reduce stress 35. A recent report found 1 in 5 professional football players within the English Football league currently use nicotine pouches or snus 35. We observed that sublabial administration of the nicotine pouch or Snus led to a mean 13% increase in mean glucose concentrations up to 55 minutes post-administration. However, peak change was variable for each administration and participant as displayed by Figure 3. Nicotine increases lipolysis and theories surrounding this response relate to it potentially being driven through the inhibition of glucose uptake 36, 37.
Current recommendations emphasise the importance of rapid glycogen resynthesis within the first four hours post-match, with inadequate glycogen resynthesis leading to a reduction in performance for the next 48 - 72 hours 34. As such, the novel findings from this study appear to suggest a contributing role of nicotine interfering with glucose regulation, which could influence both glucose stability and glycogen resynthesis, with the potential for negative effects on recovery. Given the widespread use of nicotine pouches and Snus in both professional and amateur football 35, further research is required to determine the effects of nicotine on blood glucose in a larger population and under controlled conditions.
Practical Considerations
Although CGM successfully recorded blood glucose on 98% of occasions, careful consideration is required on whether the performance insights gained outweigh the device cost and analysis time. However, there is value in discovering potentially unexpected changes in glucose concentrations not linked to diet or exercise, such as those seen in the current study with nicotine pouch/Snus use. Such observations may extend to pre- and post-match individualised carbohydrate recommendations and periodisation. These observations increase awareness and may enable support staff to implement preventative strategies.
CGM may increase player awareness of how diet and lifestyle practices influence their glucose concentrations, which could be used as a tool to encourage positive change. In the present study, carbohydrate intake in match day -1, was observed to be considerably lower than UEFA recommendations (observed 4.2 ± 0.9 g/kg BM, UEFA guidance 6-8 g/kg) 34, which is consistent with previous studies in football players 38, 39. The present study observed a modest non-significant increase in CHO intake during CGM of 0.2 g/kg BM or 20 g per day. Despite this, an argument could be made that within the applied setting this tentatively supports CGM use to positively change lifestyle behaviours, as has been previously shown in clinical populations 32.
Some issues that may impact feasibility were identified. CGM are currently targeted for clinical use and include a built-in upper limit to prevent self-diagnoses of diabetes. In the present study, one of the six participants regularly reached/exceeded the maximal reading of 11.2 mmol/L. This is a high value that would not be expected to be regularly exceeded in a professional sport context. The inability to conduct minute-by-minute analysis during training and match-play, caused by infrequent Bluetooth connection between the device and the user’s smartphone significantly limits the depth of data interpretation. It is unclear whether glucose measurements taken every 15 minutes in these dynamic environments provide valuable insights for practitioners, given the rapid fluctuations in glucose levels. More consistent, real-time monitoring may be required to fully understand the physiological changes and provide meaningful guidance for performance and health management.
Conclusion
This study found that CGM can be feasibly used to monitor glucose concentrations in professional footballers. As with other sports, variability in glucose response was observed between participants, indicating that the insight provided by CGM could be valuable for informing personalised fuelling and recovery strategies. There may also be educational value to CGM, both to enhance players understanding of the relationship between dietary intake, glucose availability and performance, and for practitioners to identify practices that may negatively affect glucose regulation (e.g., nicotine administration).
Acknowledgments
The authors would like to thank the participants of this study for their time, effort, and co- operation during of this study.
Author contribution (according to the CRediT):
Conceptualization
Dr Ian Varley, Dr David Clayton, Ross Burbeary. Data curation – Sophie Harries
Formal analysis
Sophie Harries
Funding acquisition
Dr Ian Varley, Dr David Clayton, Ross Burbeary Investigation – Sophie Harries
Methodology
Sophie Harries, Dr Ian Varley, Dr David Clayton, Ross Burbeary Resources – Dr Ian Varley, Dr David Clayton, Dr Michael Johnson, Ross Burbeary Supervision – Dr Ian Varley, Dr David Clayton, Dr Michael Johnson, Dr Robin Thorpe Validation – Sophie Harries, Dr Ian Varley, Dr David Clayton, Dr Michael Johnson Visualization – Sophie Harries, Dr Ian Varley, Dr David Clayton, Dr Michael Johnson
Writing – original draft
Sophie Harries, Dr Ian Varley, Dr David Clayton, Dr Michael Johnson
Writing – review & editing
Sophie Harries, Dr Ian Varley, Dr David Clayton, Dr Michael Johnson, Ross Burbeary, Dr Robin Thorpe.
Funding details
Funding for this project was provided by Nottingham Trent University as part of a PhD studentship.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are openly available in Zenodo at https://doi.org/10.5281/zenodo.14202932
Code availability statement
The code that support the statistical analysis of this study are openly available in Zenodo at https://doi.org/10.5281/zenodo.14202932.
Ethics approval and Informed Consent
Ethics approval was granted from Nottingham Trent University (1849189).
References
- 2.Krustrup P. (2011) Maximal voluntary contraction force, SR function and glycogen resynthesis during the first 72 h after a high-level competitive soccer game’,Eur. , J. Appl. Physiol 111, 2987-2995.
- 3.Knuiman P, Hopman M T E, Mensink M. (2015) Glycogen availability and skeletal muscle adaptations with endurance and resistance exercise’,Nutr.Metab. 12, 59.
- 4.J F Hudson, M, D J Owens, J P Morton, G L Close et al. (2021) Fuel for the Damage Induced”: Untargeted Metabolomics in Elite Rugby Union Match Play’,Metabolites. 11, 10-3390.
- 5.T K Khong, V S Selvanayagam, S K, Yusof A. (2017) Role of carbohydrate in central fatigue: a systematic review’,Scand. , J. Med. Sci. Sports 27, 376-384.
- 6.Flockhart M, F J Larsen. (2023) Continuous Glucose Monitoring in Endurance Athletes: Interpretation and Relevance of Measurements for Improving Performance and Health’,Sports Med. 10-1007.
- 7.Sylow L, Kleinert M, E A Richter, T E Jensen. (2017) Exercise-stimulated glucose uptake — regulation and implications for glycaemic control’,Nat. , Rev. Endocrinol 13, 133-148.
- 8.A D Karelis, Smith J E W, D H Passe, Péronnet F. (2010) . , Carbohydrate Administration and Exercise Performance’,Sports Med 40, 747-763.
- 9.M. (2022) da Conceição Chaves de Lemos, ‘Carbohydrate Counting’, inEndocrinology and Diabetes: A Problem Oriented Approach. , Eds., Cham: 425-431.
- 10.R J Galindo, Aleppo G. (2020) Continuous glucose monitoring: The achievement of 100 years of innovation in diabetes technology’,Diabetes Res. , Clin.Pract 170, 108502.
- 11.Oliver N. (2019) Continuous Glucose Monitoring in People With Type 1 Diabetes on Multiple -Dose Injection Therapy: The Relationship Between Glycemic Control and Hypoglycemia’,Diabetes Care. 43, 53-58.
- 12.Holzer R, Bloch W, Brinkmann C. (2022) Continuous Glucose Monitoring in Healthy Adults—Possible Applications in Health Care. , Wellness, and Sports’,Sensors 22, 10-3390.
- 13.Ishihara K, Uchiyama N, Kizaki S, Mori E, Nonaka T et al. (2020) Application of Continuous Glucose Monitoring for Assessment of Individual Carbohydrate Requirement during Ultramarathon Race’,Nutrients. 12, 10-3390.
- 14.Sengoku Y, Nakamura K, Ogata H, Nabekura Y, Nagasaka S et al. (2015) Continuous Glucose Monitoring During a 100-km Race: A Case Study in an Elite Ultramarathon Runner’,Int. , J. Sports Physiol. Perform 10, 124-127.
- 15.A-L M Bowler, Whitfield J, Marshall L, V G Coffey, L M Burke et al. (2022) The Use of Continuous Glucose Monitors in Sport: Possible Applications and Considerations’,Int. , J. SportNutr.Exerc.Metab 33, 121-132.
- 16.Diabetes American. (2018) Association, ‘Good to Know: Factors Affecting Blood Glucose’,Clin. , Diabetes 36, 202.
- 17.N S Guest, Horne J, S M Vanderhout, El-Sohemy A. (2019) Sport Nutrigenomics: Personalized Nutrition for Athletic Performance’,Front.Nutr. 6, 10-3389.
- 18.Wati I D P, N W Kusnanik, E S Wahjuni, Samodra Y T J, M F Gandasari et al. (2024) Eat well to the best performance: calorie intake and eating behavior among athlete: a review’,Int. , J. Public Health Sci. IJPHS 13, 253.
- 19.Nay K. (2019) Gut bacteria are critical for optimal muscle function: a potential link with glucose homeostasis’,Am. , J. Physiol.-Endocrinol.Metab 317, 158-171.
- 20.G A Brooks. (2020) The Precious Few Grams of Glucose During Exercise’,Int. , J. Mol. Sci 21, 10-3390.
- 21.UCI ‘General. (2024) Oranisation of Cycling as a Sport’. [Online]. Available: https://assets.ctfassets.net/761l7gh5x5an/wQympSG6EWlKq6o6HKw9E/dbf22e49f7cde9ae61df6180c7a90c8e/1-GEN-20240617_E.pdf , E0624
- 22.Hiromatsu C, Kasahara N, Lin C-A, Wang F, Goto K. (2023) Continuous Monitoring of Interstitial Fluid Glucose Responses to Endurance Exercise with. , Different Levels of Carbohydrate Intake’,Nutrients 15, 10-3390.
- 23.P S Bradley. (2013) Match performance and physical capacity of players in the top three competitive standards of English professional soccer’,Hum. , Mov. Sci 32, 808-821.
- 24.Alva S. (2022) Accuracy of a 14-Day Factory-Calibrated Continuous Glucose Monitoring System With Advanced Algorithm. in Pediatric and Adult Population With Diabetes’,J. Diabetes Sci. Technol 16, 70-77.
- 25.Abbott.Home | FreeStyle Libre | Abbott’. Accessed: Available:. https://www.freestyle.abbott/uk-en/home.html
- 27.Costello N, Deighton K, Dyson J, Mckenna J, Jones B. (2017) Snap-N-Send: A valid and reliable method for assessing the energy intake of elite adolescent athletes’,Eur. , J. Sport Sci 17, 1044-1055.
- 28.Bangsbo J, Mohr M, Krustrup P. (2006) Physical and metabolic demands of training and match-play in the elite football player’,J. , Sports Sci 24, 665-674.
- 29.A-L M Bowler, L M Burke, V G Coffey, G R Cox. (2024) Day-to-Day Glycemic Variability Using Continuous Glucose Monitors. in Endurance Athletes’,J. Diabetes Sci. Technol 19322968241250355.
- 30.A T Hulton, J, N D Clarke, MacLaren D P M. (2022) Energy Requirements and Nutritional Strategies for Male Soccer Players: A Review and Suggestions for Practice’,Nutrients. 14, 10-3390.
- 31.Russell M, Kingsley M. (2014) . , The Efficacy of Acute Nutritional Interventions on Soccer Skill Performance’,Sports Med 44, 957-970.
- 32.Ehrhardt N, Al E. (2019) Zaghal, ‘Behavior Modification in Prediabetes and Diabetes: Potential Use of Real-Time Continuous Glucose Monitoring’,J. , Diabetes Sci. Technol 13, 271-275.
- 33.Chryssanthopoulos C. (2024) Dietary Intake of Soccer Players before, during and after an Official Game:. , Influence of Competition Level and Playing Position’,Nutrients 16, 10-3390.
- 34.Collins J. (2021) UEFA expert group statement on nutrition in elite football. Current evidence to inform practical recommendations and guide future research’,Br. , J. Sports Med 55, 416-416.
- 35.Read D, Cope E, Taylor L. English Professional Football’, Loughborough University (2024) Snus Use In. [Online]. Available: https://www.lborolondon.ac.uk/media/media/london/images/news/2024/snus-use-in-english-professional-football.pdf
- 36.B C. (2012) Novel and Reversible Mechanisms of Smoking-Induced Insulin Resistance in Humans’,Diabetes. 61, 3156-3166.
- 37.Carlsson S. (2017) Smokeless tobacco (snus) is associated with an increased risk of type 2 diabetes: results from five pooled cohorts’,J. , Intern. Med 281, 398-406.