Lipopolysaccharide Prompts Oxidative Stress and Apoptosis in Rats’ Testicular Tissue
Abstract
Lipopolysaccharide (LPS) is a component of the outer membrane of gram negative bacteria. LPS challenging allows switching transcription of proinflammatory cytokines on via over stimulation of Toll-like receptors (TLRs) signaling pathway with subsequent pathogenic inflammatory response. We investigated the possible reproductive toxicity of LPS in male Wister albino rats. Oxidative stress markers, antioxidant status and caspase-3 activity were analyzed in testicular tissues of rats exposed to either saline or LPS (4 mg/kg BW, ip; 0.18 of the LD50). The samples were collected at 6 h and 72 h after injection of LPS. A significant reduction in testicular reduced glutathione (GSH), glutathione-S-transferase (GST) and superoxide dismutase (SOD) was observed at 72 h compared to control group. Total antioxidant capacity was decreased at 6 h with additional significant reduction at 72 h. Catalase activity was reduced significantly at both 6 and 72 h. Malondialdehyde (MDA) was increased (P ≤ 0.05) in LPS injected rats without variation between 6 and 72 h. A significant increase in nitric oxide (NO) was observed at 72 h after injection. A time-dependent increase in LPS-treated groups was observed in the concentration of caspase-3.Histopathological analysis revealed degenerative changes and necrosis of seminiferous tubules after 6 h with further accumulation of eosinophilic edematous transudate in its lumen after 72 h. In conclusion, by increasing time of exposure, LPS induced lipid peroxidation, oxidative stress, reduced testicular antioxidant capacity and encouraged testicular apoptosis which could be possible mechanisms for impairment of testicular function.
Author Contributions
Academic Editor: BURAK DiK, Selcuk University, Veterinary Faculty, Farmacology And Toxicology, TURKEY
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2018 Amal A Halawa, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Lipopolysaccharide (LPS) is a glycolipid component of the cell wall of gram negative bacteria such as Escherichia and Salmonella species. The name of endotoxin is due to the biological activities that induced by LPS after entering the host organism 1. LPS consists of lipid A part which is responsible for the toxic proinflammatory properties of LPS 2,3 and polysaccharide side chain part, which consists of a core oligosaccharide and the terminal O-specific chain antigen 4, 5 that determines the serological specificity or the bacterial serotype 6. LPS is considered as a molecular pattern related to the pathogen-associated molecular patterns (PAMPs] via which the bacteria can be recognized by specific host receptors called pattern recognition receptors (PRRs) 7. LPS performs as a toxin via over stimulation of Toll-like receptors (TLRs)signaling pathway, that promotes nuclear translocation of NF-kβ 8 and switches on transcription of proinflammatory cytokines such as IL-1β, IL-6 and TNFα that encourages pathogenic inflammatory responses 5,9. Exposure to high doses of LPS triggers the production of the proinflammatory mediators which may result in a harmful condition termed oxidative stress 10,11. Reactive oxygen species (ROS) are thought to be involved in the mechanism of LPS toxicity 12.
Male infertility is essentially a result of disruption in spermatogenesis process, which is based onreduction in sperm count, motility and viability, oxidative stress and disturbance in sex hormones 13. Infections lead to an extreme production of ROS, resulting in an oxidative stress which impairs the sperm functions, as well as fertilization 14. It was suggested that LPS inhibits human sperm motility by decreasing intracellular cAMP 15. Previous study evaluated the adverse effects of LPS on male reproduction system identifying increased oxidative stress and proinflammatory stress markers in the testes leading to marked testicular dysfunction 16.
We aimed in the present study to estimate the potential mechanisms for LPS to induce testicular dysfunction via measuring the antioxidant activity and oxidative stress, in addition the effect of LPS on the apoptotic gene marker, Caspase-3 in male albino rats. Histopathological analysis on testicular structure was also applied.
Materials and Methods
Chemicals
Lipopolysaccharide (LPS) (E. coli, serotype 0111:B4; Sigma-Aldrich) was dissolved in distilled water. Rats in control group were administered intraperitoneal pyrogen-free 0.9% NaCl.
Animals
Fifteen male Wister albino rats (3 months old) were purchased from Faculty of Medicine, Mansoura University, Mansoura, Egypt. The animals were allowed for accommodation at 2 weeks for the laboratory conditions (12 h light/dark cycles) before commencement of the experiment. Animals were received feed and water ad libitum. Animals received human care in compliance with the guidelines of animal care of the National Institutes of Health, and all animals applications were performed in accordance with the Ethics Committee of the National Research Centre, Egypt, registration number (09/189).
Experimental Design
A total of 15 rats were divided into control (n = 5) and LPS treated groups (n = 10). In the second group, testes were collected after 6 h (n=5) and after 72 h (n=5). Control rats received intraperitoneal injection of pyrogen-free 0.9% NaCl. According to Nezić et al (2009) 17, single intraperitoneal injection of non-lethal dose of LPS was administered to the group treated with LPS (4 mg/kg BW; 0.18 of the LD50 22.15 mg/kg) 17 Rats were sacrificed and testes were collected at 6 and 72 h time points after LPS injection.
Tissue Preparation
One g of each testis was washed by PBS (pH 7.4) and then homogenized in nine volume of PBS. Tissue homogenate was further centrifuged at 4000 rpm for 20 minutes at 4⁰C and stored at -80⁰C according to Ferdandez-botran et al. (2002) 18 till antioxidant and oxidative stress markers were performed.
Biochemical Analysis:
Testicular Reduced Glutathione Concentration:
Reduced glutathione in testicular homogenate was determined using the method of Beutler et al. (1963) 19. 5, 5 dithiobis 2- nitrobenzene (DTNB) was used to give yellow colored complex in conjugation with reduced glutathione
Testicular Glutathione-S-tTransferase Activity:
GST activity was determined by measuring the conjugation of 1- chloro- 2, 4-dinitrobenzene (CDNB) with reduced glutathione according to method of Habig et al. (1976) 20.
Testicular Superoxide Dismutase Activity:
Tissue homogenate of testis was mixed with working solution contained with 0.5mM phosphate buffer, 0.3mM NADPH+ and 0.5mM nitroblue tetrazolium for a min then the reaction was further inhibited by phenazine methosulphate. The percent of inhibition of superoxide radical was estimated and multiplied by 3.75 to determine SOD activity according to Nishikimi et al. (1972) 21.
Total Antioxidant Capacity:
Capacity of total antioxidant capacity was determined according to Koracevic et al. (2001) 22. Capacity of total antioxidant was determined with conjugation of hydrogen peroxide with 3,5,dichloro-2-hydroxy benzensulphonate to form pinky color compound that inversely proportional with total antioxidant capacity of samples.
Testicular Catalase Activity:
According to Aebi (1984) 23, catalase activity was determined through the enzymatic reaction initiated via binding of the produced hydrogen peroxide with 3,5-Dichloro-2-hydroxybenzene sulphonic acid and 4-aminophenazone. The produced colored complex is inversely proportional with catalase activity in tissues.
Oxidative Stress Marker (Lipid peroxidation):
The levels of lipid peroxidation expressed as malondialdehyde concentration was measured spectrophotometrically followed the method of Draper and Hadley (1990) 24. Thiobarbituric acid reacts with MDA in acid medium to form reactive product. The absorbance of the resultant pink product can be measured at 534 nm.
Nitrosative Stress Marker:
The level of nitrite was used to determine the extent of nitrosative stress on testicular tissues according to the method of Montgomery and Dymock (1961) 25. In the presence of nitrite and in an acidic medium, the formed nitrous acid diazotized sulphanilamide and the product is coupled with N-(1-naphthyl) ethylenediamine. The resulting azodye has a bright reddish-purple color which can be measured at 540 nm.
Flowcytometric Determination of Caspase 3:
The determination of caspase as an apoptotic marker was occurred using flow cytometric technique according to technique of Tribukait (1987) 26. Testicular tissues were washed in isotonic tris EDTA buffer (3.029 g of 0.1 M tris (hydroxymethyl aminomethane , 1.022 g of 0.07 M sodium chloride and 0.47 g of 0.005 M EDTA) and were further suspended in absolute ethanol for accomplishing of fixation step of testicular tissues. The fixed tissues was conjugated by adequate dilution (1:50) of caspase-3 primary antibody (Abcam, USA), that was further mixed with secondary antibodies (IgG) (Abcam, USA) with the appropriate dilution (1:2000). Finally, cells were suspended in solution containing PBS/BSA. After staining procedures were occurred, measurements of positive cells was determined using flow cytometer device (FACS caliber flow cytometer "Becton Dickinson , Sunnyvale , CA, USA) equipped with a compact air cooked low power 15 mwatt argon iron laser beam (488 nm).
Histopathological Examination:
The testes were dehydrated in graded ethanol and embedded in paraffin wax. 5 μm thick paraffin embedded sections were cut and routinely stained with hematoxyline and eosin according to Bancroft et al (1996) 27. Each section was examined by light microscopy.
Statistical Analysis:
The results were expressed using one way ANOVA to determine the difference between means in every group. Least significance difference using Duncan’s post-hoc test was used to determine statistically significance difference between means at a significant level 0.05 where P ≤ 0.05. All data were reported as means ± SEM. Statistical analyses were done using SAS® (version 9.2, SAS Institute, Cary, NC, USA). For all analyses, P ≤ 0.05 was defined as significant.
Results
Effects of LPS on Antioxidant Activity
Antioxidant Status:
Glutathione concentration (GSH) showed a significant reduction at 72 h (15.42 ± 0.678 mg/g) after LPS injection compared to 6 h (17.58 ± 0.174 mg/g) exposure and control (18.17 ± 0.593 mg/g) groups (Figure 1a). GST showed a significant decrease after 72 h (0.677 ± 0.009 U/g) of LPS exposure in comparison with control (1.343 ± 0.015 U/g) and 6 h (0.967 ± 0.152 U/g) exposure (P ≤ 0.05) (Figure 1b) . SOD activity was reduced at 6 h (238.82 ± 7.78 U/g) without significant variation compared to control (242.13 ± 7.83 U/g) while the reduction at 72 h (207.81 ± 8.14 U/g) was significantly varied compared to control and 6 h groups (P ≤ 0.05) (Figure 1c).
Figure 1.a) GSH concentration, b) GST activity and c) SOD activity in control and LPS-treated groups (4 mg/kg BW ip). Results are expressed as mean ± SEM. The different letters are statistically significant (P ≤ 0.05).
Total Testicular Antioxidant Capacity:
A significant reduction in total antioxidant capacity was observed at 6 h (0.66 ± 0.017μmol/g) with additional decline at 72 h (0.203 ± 0.026 μmol/g) compared to control group (0.947 ± 0.009 μmol/g) (P ≤ 0.05) (Figure 2a). Corresponding to antioxidant capacity of rats exposed to LPS, the activity of catalase was decreased significantly at 6 h (0.187 ± 0.035 U/g) and 72 h (0.17 ± 0.012 U/g) compared to control group (0.317 ± 0.019 U/g) (P ≤ 0.05) without significant variation between LPS-treated groups (Figure 2b).
Figure 2.a) Total antioxidant capacity and b) Catalase activity in control and LPS-treated groups (4 mg/kg BW ip). Results are expressed as mean ± SEM The different letters are statistically significant (P ≤ 0.05).
Oxidative Stress Marker:
The levels of lipid peroxidation (MDA) in rats exposed to LPS showed a significant increase after 6 h (12.41 ± 0.49 nmol/g) and 72 h (10.62 ± 0.50 nmol/g) of exposure in comparison with control group (7.38 ± 0.50 nmol/g) (P ≤ 0.05). Although there was a reduction in testicular MDA levels after 72 h compared to 6 h, but this reduction was non-significant (P > 0.05) (Figure 3a).
Figure 3.a MDA concentration and b) NO concentration in control and LPS-treated groups (4 mg/kg BW ip). Results are expressed as mean ± SEM. The different letters are statistically significant (P ≤ 0.05).
Nitrosative Stress Marker:
A robust increase occurred (P ≤ 0.05) in testicular nitrite levels after 72 h (35.4 ± 1.12 nmol/g) after LPS injection compared to control group (25.61 ± 3.45 nmol/g). The increase of nitrite level at 6 h was insignificant (31.2 ± 0.46 nmol/g) (Figure 3b).
Effect of LPS on Apoptotic Factor Caspase -3
The concentration of caspase-3 showed a marked increase (P ≤ 0.05) at either 6 h (32.24 ± 2.15) or 72 h (54.74 ± 3.29) compared to control group (15.12 ± 1.22) Figure 4.
Figure 4.Relative expression of caspase-3 in testicular tissues of either control or LPS-treated rats (4 mg/kg BW ip). Results are expressed as mean ± SEM. The different letters are statistically significant (P ≤ 0.05).
Histopathological Analysis of Testicular Tissue
Seminiferous tubules showed normal spermatogenic epithelium, Sertoli cells and interstitial tissue in control group (Figure 5a), whereas testes collected 6 h after LPS injection displayed degenerative changes and necrosis of seminiferous tubules (Figure 5b). After 72 h, necrosis of spermatogenic epithelium lining seminiferous tubules and accumulation of eosinophilic edematous transudate in its lumen were observed (Figure 5c).
Figure 5.Histopathological results. a) Control group: seminiferous tubules with normal spermatogenic epithelium (arrow), normal Sertoli cells and normal interstitial tissue. b) After 6 hours seminiferous tubules display degenerative changes and necrosis of spermatogenic epithelium (arrow) and abnormal Sertoli cells. c) After 72 h necrosis of spermatogenic epithelium lining seminiferous tubules and accumulation of eosinophilic edematous transudate in its lumen.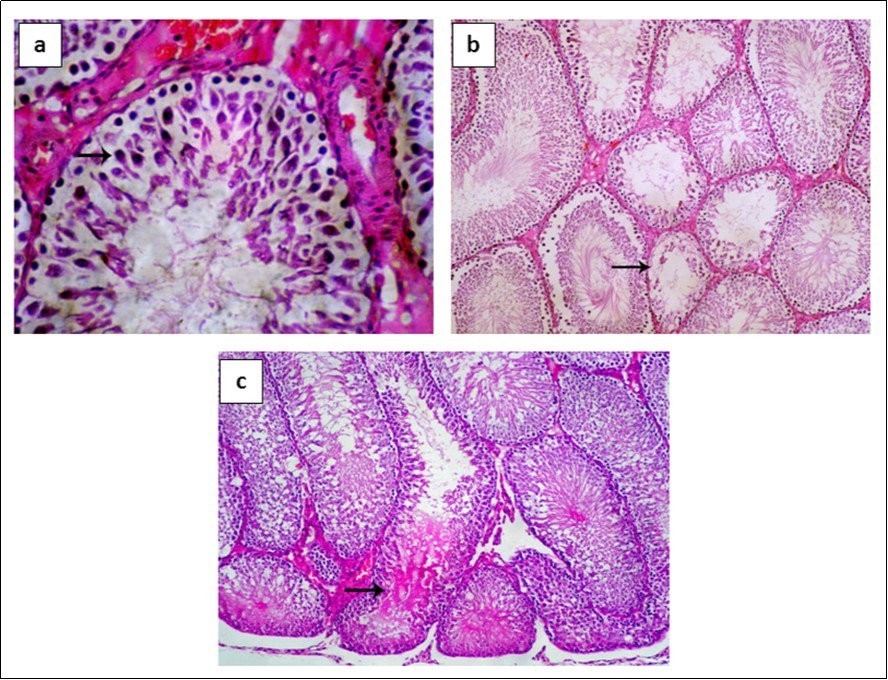
Discussion
Testicular tissues may lack an adequate oxygen supply, so testes may suffer dramatically from oxidative stress due to the high levels of unsaturated fatty acids and excessive production of ROS that could be produced from leakage of mitochondrial membrane and might impact both spermatogenic and steroidogenic functions 28, 29. At the testicular level, oxidative stress can be modulating steroidogenic capacity of Leydig cells 30 besides disrupting ability of germinal epithelium to differentiate spermatozoa 31 which may result in infertility 32.
The capability of LPS for induction of oxidative stress in testicular tissues was previously studied in mice 33. It was reported that the LPS could possibly induce oxidative stress in rats due to the disruption in germ cell layer in seminiferous tubules which increased the production of proinflammatory cytokines and generation of ROS 34. Moreover, the LPS would possibly increase the activity and concentration of nitric oxide synthetase (NOS) in testes that produced excessive amount of nitric oxide causing nitrosative stress in adult rats 35. We aimed to reveal the ability of LPS to induce oxidative stress as well as apoptosis in short periods of time (6 and 72 h) in testicular tissue of rats as possible mechanisms for testicular dysfunction.
In the current study, GSH concentration showed a significant decrease after 72 hours of LPS treatment, and occured a decrease in GST activity in comparison with control group. It was shown the induced infection using LPS was capable of encouraging oxidative stress that concurrently would result in decrease GSH in human dendritic cells 36. In another study, GST activity showed a significant decrease after Cyanobacterium LPS treatment in zebra fish 37. Similarly, the activity of hepatic GST showed a significant decline when galactosamine /lipopolysaccharide were injected to rats 38.
As a result of the oxidative damage and generation of free radicals, the concentration of total antioxidant capacity revealed a significant gradually reduction by the increase with time of LPS exposure. In a similar study, rats administered 30 mg/kg LPS showed a significant reduction in total antioxidant capacity that was measured as ferric reducing ability of plasma 39. The reduction of antioxidant capacity was followed by a significant decrease in testicular catalase activity by 54% after 72 hours of treatment which was further confirmed by the study of Spolarics and Wu (1997) 40, whom found that LPS treatment would result in a significant reduction in catalase activity by 45% in Kupffer cells 40. Such reduction reflects the inability of testicular cells to eliminate H2O2 produced by the inflammatory events resulting from LPS injection or may be due to enzyme activation caused by ROS generated in testicular cells a41, 42.
SOD activity showed a significant decrease after 72 hours of LPS treatment which was observed in broilers treated with 0.5 mg/ml LPS attributing to disturbance in antioxidant defense system 43.
One of the various effects of oxidative stress is peroxidation of unsaturated fatty acids in membrane. The degree of lipid peroxidation, measured as MDA, was used to explain the role of LPS in peroxidation of lipids in testicular tissues. The concentration of MDA was increased immediately after 6 h of LPS administration with slight non-significant reduction at 72 h. Testicular MDA levels increased at the same dose of LPS at 6 h and returned to normal level at 12 and 72 h 44. Likewise, 5 mg/kg LPS increased MDA levels in albino rats with reduction in testicular antioxidant activities 41. A robust increase the levels of lipid peroxidation were observed with higher doses of LPS in Fischer rats 45. Also, LPS (200 μg/mouse, for 2 h) induced lipid peroxidation products (MDA and 4-hydroxynonenal) in isolated mice Leydig cell homogenates 46. Moreover, LPS- treated Sertoli cells of adult rats (50 μg/ml for 12 h) showed augmentation in H2O2 and lipid peroxidation with reduction in activities and concentration of the antioxidant parameters (GSH, GST, SOD, CAT) 47. Ability of LPS (1 μg/ml, for 12 h) to exert oxidative stress was also verified using primary culture of rat Leydig cells 48.
The levels of nitrosative stress were determined using the levels of nitric oxide production in testicular tissues. NO was increased significantly after 72 h of treatment which was observed as well in macrophage cell line due to induction of nitric oxide synthetase expression in macrophage 49. In a study performed by O'Bryan et al. (2000) 50, testicular tissues of rats showed a significant increase in NO production due to up-regulation of inducible nitric oxide synthetase (iNOS) after LPS administration 50.
Stangelet al. (1996) 51 found that ROS and NO were capable of inducing apoptosis in rat skeletal myoblast 51. Apoptosis is a programmed cell death which occurs physiologically in testicular germ cells to balance their number to that of Sertoli cells 52. Apoptosis is generated by various pathways, all initiate Caspase pathway (Caspase 8 and 9) that in turn activate the effector Caspase 3 53, 54. The results of the present study showed early induction of apoptosis through increase in Caspase-3 concentration (6 h), which could be due to the induction of testicular oxidative stress as well as increase in NO levels. Pro-inflammatory cytokines that were induced by LPS is another mechanism by which apoptosis could be induced through TNF-dependent pathway 55. Besides, LPS was capable of inducing apoptosis in hepatocyte which was followed by an increase in production of Caspase-3 in co-culture of hepatocyte and Kupffer cells 56. LPS administered to mice (intraperitoneal, 0.1 mg/kg, 7 days) caused increase of apoptotic germ cells via Fas/ FasL system up to 5 weeks after LPS exposure and observed peak at 24 h 57. Additionally, Leydig cells incubated for 12 h with LPS was showed increase in Caspase 3 activity 58.
The aforementioned alterations that were observed in the present work were supported by the histopathological examination of the testicular tissue. After 6 h, seminiferous tubules showed necrosis and degenerative changes, meanwhile, testes collected 72 h post LPS injection, showed necrosis of spermatogenic epithelium lining seminiferous tubules with eosinophilic edematous transudate in its lumen. Similarly, at the same dose of LPS, accumulation of immature germ cells in the lumen of seminiferous tubules was observed after 6 h of LPS injection. While at 72 h, large numbers of immature germ cells were observed in the lumen of epididymis with intercellular gaps in seminiferous epithelium 44. The histopathological lesions in rat testes after single intraperitoneal injection of LPS revealed normal structure of seminiferous tubules at 3 and 6 h after LPS injection; whereas seminiferous epithelium showed degeneration and sloughing at 24 and 72 h time points 41. At the same dose of LPS, seminiferous tubules showed thinning in its epithelium lining disordered architecture and uneven arrangement of spermatogenic cells, together with reduced number of mature sperms and noticeable shedding of spermatogenic cells in the lumens of seminiferous tubules were observed. Such lesions become more pronounced by 72 h after LPS injection 59.
The data of the current work are in consistence with other studies proved that oxidative stress is considered as a key factor in steroidogenesis and spermatogenesis alteration with subsequent infertility 41, 50, 60.
In conclusion LPS can induce oxidative stress after short period from exposure as early as 6 h and continue up to 72 h resulting from a significant decrease in antioxidant defense enzymes with an increase in lipid peroxidation and NO levels. The LPS-induced oxidative stress could be accompanied with apoptosis through activation of Caspase-3 resulting in impairment in testicular functions. This effect is a crucial for the reproductive performance and future fertility of male due to the LPS effect on germinal epithelium of testes.
Funding
The author(s) received no financial support.
Acknowledgements
The authors appreciate everyone participate in the present work.
References
- 1.D S Kabanov, I R Prokhorenko. (2010) Structural analysis of lipopolysaccharides from Gram-negative bacteria. , Biochem 75, 383-404.
- 2.Raetz C R H, T A Garrett, C M Reynolds, W A Shaw, J D Moore. (2006) Kdo2-Lipid A of Escherichia coli, a defined endotoxin that activates macrophages via TLR-4. , J. Lipid Res 47, 1097-1111.
- 3.C J Kirschning, Bauer S. (2001) Toll-like receptors: cellular signal transducers for exogenous molecular patterns causing immune responses. , Int. J. Med. Microbiol 291, 251-260.
- 4.J C Hodgson. (2006) Endotoxin and mammalian host responses during experimental disease. , J. Comp. Pathol 135, 157-175.
- 5.J E Tomlinson, A T Blikslager. (2004) Interactions between lipopolysaccharide and the intestinal epithelium. , J. Am. Vet. Med. Assoc 224, 1446-1452.
- 6.Alexander C, Rietschel E. (2001) Bacterial lipopolysaccharides and innate immunity. , Endotoxin. Res 7, 167-202.
- 7.Eckmann L. (2004) Innate immunity and mucosal bacterial interactions in the intestine. , Curr. Opin. Gastroenterol 20, 82-88.
- 8.H D Brightbill, R L Modlin. (2000) Toll-like receptors: molecular mechanisms of the mammalian immune response. , Immunology 101, 1-10.
- 9.Gutsmann T, A B Schromm, Brandenburg K. (2007) The physicochemistry of endotoxins in relation to bioactivity. , Int. J. Med. Microbiol 297, 341-352.
- 10.Libby P. (2007) Inflammatory mechanisms: the molecular basis of inflammation and disease. , Nutr. Rev 65, 140-146.
- 11.Victor V M, Rocha M, De la Fuente, M. (2004) Immune cells: free radicals and antioxidants in sepsis. , Int. Immunopharmacol 4, 327-347.
- 12.Kallapura G, N R Pumford, X Hargis Hernandez-Velasco, B M Tellez, G. (2014) Mechanisms involved in lipopolysaccharide derived ROS and RNS oxidative stress and septic shock. , J. Microbiol. Res. Rev 2.
- 13.Sikka S C. (2001) Relative impact of oxidative stress on male reproductive function. , Curr. Med. Chem 8, 851-862.
- 14.F R Ochsendorf. (1999) Infections in the male genital tract and reactive oxygen species. , Hum. Reprod. Update 5, 399-420.
- 15.Li Z, Zhang D, He Y, Ding Z, Mao F. (2016) Lipopolysaccharide Compromises Human Sperm Function by Reducing Intracellular cAMP. , Tohoku J. Exp. Med 238, 105-112.
- 16.Abd-Allah A R, Helal G K, Al-Yahya A A, Aleisa A M, Al-Rejaie S S. (2009) Pro-inflammatory and oxidative stress pathways which compromise sperm motility and survival may be altered by L-carnitine. , Oxid. Med. Cell Longev 2, 73-81.
- 17.Nezić L, Skrbić R, Dobrić S, Stojiljković M P, Satara S S. (2009) Effect of simvastatin on proinflammatory cytokines production during lipopolysaccharide-induced inflammation in rats. , Gen. Physiol. Biophys 28, 119-126.
- 18.Ferdandez-botran R, Gorantala V, Sun X, Ren X, Perez-Abadia G. (2002) Targeting of glycosaminoglycan-cytokine interactions as a novel therapeutic approach in allotransplantation. , Transplantation 74, 623-629.
- 19.Beutler E, Duron O, Kelly B. (1963) Improved method for the determination of blood glutathione. , J. Lab. Clin. Med 61, 882-888.
- 20.Habig W H, Pabst M J, Jakoby W B. (1976) Glutathione S-transferase AA from rat liver. , Arch. Biochem. Biophys 175, 710-716.
- 21.Nishikimi M, N A Rao, Yagi K. (1972) The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. , Biochem. Biophys. Res. Commun 46, 849-854.
- 22.Koracevic D, Koracevic G, Djordjevic V. (2001) Method for the measurement of antioxidant activity in human fluids. , J. Clin. Pathol 54, 356-361.
- 24.Draper H, Hadley M. (1990) Malondialdehyde determination as index of lipid peroxidation. , Methods Enzymol 186, 421-431.
- 26.Tribukait B. (1987) Flow cytometry in assessing the clinical aggressiveness of genito-urinary neoplasms. , World J. Urol 5, 108-122.
- 27.Bancroft J D, Stevens A. (1996) Theory and practice of histological technique. 4th Ed , Churchill, Livingston, Edinburgh, London, Melbourne, NY .
- 28.Kumagai A, Kodama H, Kumagai J, Fukuda J, Kawamura K. (2002) Xanthine oxidase inhibitors suppress testicular germ cell apoptosis induced by experimental cryptorchidism. , Mol. Hum. Reprod 8, 118-123.
- 29.Aitken R J, Roman S D. (2008) Antioxidant systems and oxidative stress in the testes. , Adv. Exp. Med. Biol 636, 154-71.
- 30.Hales D B, Allen J A, Shankara T, Janus P, Buck S. (2005) Mitochondrial function in Leydig cell steroidogenesis. , Ann. N Y. Acad. Sci 1061, 120-134.
- 31.Naughton C K, Nangia A K, Agarwal A. (2001) Pathophysiology of varicoceles in male infertility. , Hum. Reprod. Update 7, 473-481.
- 32.Agarwal A, Gupta S, Sikka S. (2006) The role of free radicals and antioxidants in reproduction. , Curr. Opin. Obstet. Gynecol 18, 325-332.
- 33.Rao F, Tian H, Li W, Hung H, Sun F. (2016) Potential role of punicalagin against oxidative stress induced testicular damage. , Asian J. Androl 18, 627-32.
- 34.Zhao L, Chen Y-H, Wang H, Ji Y L, Ning H. (2008) Reactive oxygen species contribute to lipopolysaccharide-induced teratogenesis in mice. , Toxicol. Sci 103, 149-157.
- 35.Jonsson C K, Setchell B P, Martinelle N, Svechnikov K, Söder O. (2001) Endotoxin-induced interleukin 1 expression in testicular macrophages is accompanied by downregulation of the constitutive expression in Sertoli cells. , Cytokine 14, 283-288.
- 36.Yamada H, Arai T, Endo N, Yamashita K, Fukuda K. (2006) LPS-induced ROS generation and changes in glutathione level and their relation to the maturation of human monocyte-derived dendritic cells. , Life Sci 78, 926-933.
- 37.Jaja-Chimedza A, Gantar M, Mayer G D, Gibbs P D, Berry J P. (2012) Effects of cyanobacterial lipopolysaccharides from microcystis on glutathione-based detoxification pathways in the zebrafish (Danio rerio) embryo. , Toxins 4, 390-404.
- 38.Abdulazeez Sheik, Thiruvengadam S, D. (2013) Effect of lycopene on oxidative stress induced during D-galactosamine/lipopolysaccharide-sensitized liver injury in rats. , Pharm. Biol 51, 1592-1599.
- 39.Skibska B, Józefowicz-Okonkwo G, Goraca A. (2006) Protective effects of early administration of alpha-lipoic acid against lipopolysaccharide-induced plasma lipid peroxidation. , Pharmacol Rep 58, 399-404.
- 40.Spolarics Z, Wu J-X. (1997) Role of glutathione and catalase in H2O2 detoxification in LPS-activated hepatic endothelial and Kupffer cells. , Am. J. Physiol 273, 1304-1311.
- 41.Reddy M M, Mahipal S V, Subhashini J, Reddy M C, Roy K R. (2006) Bacterial lipopolysaccharide-induced oxidative stress in the impairment of steroidogenesis and spermatogenesis in rats. , Reprod. Toxicol 22, 493-500.
- 42.Pigeolet E, Corbisier P, Houbion A, Lambert D, Michiels C. (1990) Glutathione peroxidase, superoxide dismutase, and catalase inactivation by peroxides and oxygen derived free radicals. , Mech. Ageing Dev 51, 283-97.
- 43.Wu Q J, Wang Y Q, Qi Y X. (2016) The protective effect of procyanidin against LPS-induced acute gut injury by the regulations of oxidative state. , Springerplus 5, 1645.
- 44.Li L, Ma P, Liu Y, Huang C, O W S. (2013) Intermedin attenuates LPS-induced inflammation in the rat testis. , PLoS One 8, 65278.
- 45.Requintina J, Oxenkrug G F. (2003) Differential effects of lipopolysaccharide on lipid peroxidation in F344N, SHR rats and BALB/c mice, and protection of melatonin and NAS against its toxicity. , Ann. N Y. Acad. Sci 993, 325-333.
- 46.Allen J A, Diemer T, Janus P, Hales K H, Hales D B. (2004) Bacterial endotoxin lipopolysaccharide and reactive oxygen species inhibit Leydig cell steroidogenesis via perturbation of mitochondria. , Endocrine 25, 265-275.
- 47.Aly H, Lightfoot D A, El-Shemy H A. (2009) Modulatory role of lipoic acid on lipopolysaccharide-induced oxidative stress in adult rat Sertoli cells in vitro. , Chem Biol Interact 182, 112-118.
- 48.Hu W, Shi L, Li M Y, Zhou P H, Qiu B. (2017) Adrenomedullin protects Leydig cells against lipopolysaccharide-induced oxidative stress and inflammatory reaction via MAPK/NF-κB signalling pathways. , Sci Rep 7, 16479.
- 49.Chang C-Y, Tucci M, Baker R C. (2000) Lipopolysaccharide-stimulated nitric oxide production and inhibition of cell proliferation is antagonized by ethanol in a clonal macrophage cell line. , Alcohol 20, 37-43.
- 50.O’Bryan M K, Schlatt S, Gerdprasert O, Phillips D J, DM de Kretser. (2000) Inducible nitric oxide synthase in the rat testis: evidence for potential roles in both normal function and inflammation-mediated infertility. , Biol. Reprod 63, 1285-1293.
- 51.Stangel M, Zettl U K, Mix E, Zielasek J, Toyka K V. (1996) H2O2 and nitric oxide-mediated oxidative stress induce apoptosis in rat skeletal muscle myoblasts. , J. Neuropathol. Exp. Neurol 55, 36-43.
- 52.Print C G, Loveland K L. (2000) Germ cell suicide: new insights into apoptosis during spermatogenesis. , Bioessays 22, 423-430.
- 53.Li X, Fang P, Mai J, Choi E T, Wang H. (2013) Targeting mitochondrial reactive oxygen species as novel therapy for inflammatory diseases and cancers. , J. Hematol.Oncol 6, 19.
- 54.Oyinloye B E, Adenowo A F, Kappo A P. (2015) Reactive oxygen species, apoptosis, antimicrobial peptides and human inflammatory diseases. Pharmaceuticals (Basel). 8, 151-175.
- 55.Baud V, Karin M. (2001) Signal transduction by tumor necrosis factor and its relatives. , Trends in Cell Biol 11(9), 372-7.
- 56.Hamada E, Nishida T, Uchiyama Y, Nakamura J, Isahara K. (1999) Activation of Kupffer cells and caspase-3 involved in rat hepatocyte apoptosis induced by endotoxin. , J. Hepatol 30, 807-818.
- 57.Kajihara T, Okagaki R, Ishihara O. (2006) LPS-induced transient testicular dysfunction accompanied by apoptosis of testicular germ cells in mice. , Med. Mol. Morphol 39, 203-208.
- 58.Zhou P H, Hu W, X B Zhang, Wang W, Zhang L J. (2016) . Protective Effect of Adrenomedullin on Rat Leydig Cells from Lipopolysaccharide-Induced Inflammation and Apoptosis via the PI3K/Akt Signaling Pathway ADM on Rat Leydig Cells from Inflammation and Apoptosis. Mediators Inflamm. http://dx.doi.org/10.1155/2016/7201549. Article ID 7201549 16.
Cited by (17)
- 1.Kumari Monika, Sharma Anamika, Tirpude Narendra Vijay, 2024, Herbacetin ameliorates lipopolysaccharide-elicited inflammatory response by suppressing NLRP-3/AIM-2 inflammasome activation, PI3K/Akt/MAPKs/NF-κB redox inflammatory signalling, modulating autophagy and macrophage polarization imbalance, Molecular Biology Reports, 51(1), 10.1007/s11033-024-10068-9
- 2.Almulla Abbas F., Supasitthumrong Thitiporn, Amrapala Arisara, Tunvirachaisakul Chavit, Jaleel Al-Karrar Kais Abdul, et al, 2022, The Tryptophan Catabolite or Kynurenine Pathway in Alzheimer’s Disease: A Systematic Review and Meta-Analysis, Journal of Alzheimer’s Disease, 88(4), 1325, 10.3233/JAD-220295
- 3.Samy Alaa, El-Adl Mohamed, Rezk Shaymaa, Marghani Basma, Eldomany Wael, et al, 2020, The potential protective and therapeutic effects of platelet-rich plasma on ischemia/reperfusion injury following experimental torsion/detorsion of testis in the Albino rat model, Life Sciences, 256(), 117982, 10.1016/j.lfs.2020.117982
- 4.Sołek Przemysław, Czechowska Ewelina, Sowa-Kućma Magdalena, Stachowicz Katarzyna, Kaczka Piotr, et al, 2023, Elucidating the molecular mechanisms underlying the induction of autophagy by antidepressant-like substances in C57BL/6J mouse testis model upon LPS challenge, Cell Communication and Signaling, 21(1), 10.1186/s12964-023-01270-6
- 5.Herrera Nicolas J, Bland Nicolas A, Ribeiro Felipe A, Henriott Morgan L, Hofferber Eric M, et al, 2021, Oxidative stress and postmortem meat quality in crossbred lambs, Journal of Animal Science, 99(7), 10.1093/jas/skab156
- 6.Abdel-Megeed Rehab M., Kadry Mai O., 2023, Amelioration of autophagy and inflammatory signaling pathways via α-lipoic acid, burdock and bee pollen versus lipopolysaccharide-induced insulin resistance in murine model, Heliyon, 9(5), e15692, 10.1016/j.heliyon.2023.e15692
- 7.Abbas Ahmed O., Alaqil Abdulaziz A., El-Beltagi Hossam S., Abd El-Atty Hanaa K., Kamel Nancy N., 2020, Modulating Laying Hens Productivity and Immune Performance in Response to Oxidative Stress Induced by E. coli Challenge Using Dietary Propolis Supplementation, Antioxidants, 9(9), 893, 10.3390/antiox9090893
- 8.Raj Vijay, Natarajan Suganya, C Marimuthu, Chatterjee Suvro, Ramasamy Mohankumar, et al, 2021, Cholecalciferol and metformin protect against lipopolysaccharide-induced endothelial dysfunction and senescence by modulating sirtuin-1 and protein arginine methyltransferase-1, European Journal of Pharmacology, 912(), 174531, 10.1016/j.ejphar.2021.174531
- 9.Suleiman Joseph Bagi, Bakar Ainul Bahiyah Abu, Mohamed Mahaneem, 2021, Review on Bee Products as Potential Protective and Therapeutic Agents in Male Reproductive Impairment, Molecules, 26(11), 3421, 10.3390/molecules26113421
- 10.Ogunro Olalekan Bukunmi, Asejeje Folake Olubukola, Hamzat Zainab Olamide, 2025, Monosodium glutamate exacerbated the lipopolysaccharide-induced reproductive toxicity of male Wistar rats, BMC Pharmacology and Toxicology, 26(1), 10.1186/s40360-025-00982-4
- 11.Sharma Kriti, Kumar Shiv, Prakash Ravi, Khanka Sonu, Mishra Tripti, et al, 2023, Chebulinic acid alleviates LPS-induced inflammatory bone loss by targeting the crosstalk between reactive oxygen species/NFκB signaling in osteoblast cells, Free Radical Biology and Medicine, 194(), 99, 10.1016/j.freeradbiomed.2022.11.026
- 12.Aboelmaaty Amal M., Omara Shimaa T., Aly Mohamed S., Kotp Mohamed S., Ali Amal H., 2022, The antibacterial and anti-inflammatory effects of zinc oxide nanoparticles synthesized by Thymus vulgaris medicinal plant against Escherichia coli and Escherichia coli lipopolysaccharides, Egyptian Pharmaceutical Journal, 21(2), 153, 10.4103/epj.epj_98_21
- 13.Koshevoy V.I., Naumenko S.V., 2022, Dynamics of peroxidation processes in male rabbits under experimental LPS-induced oxidative stress, Bulletin "Veterinary biotechnology", (41), 100, 10.31073/vet_biotech41-10
- 14.Otuechere Chiagoziem A., Adewuyi Adewale, Oluwabayo Tanitoluwa, Afolayan Folashade, Avwioroko Oghenetega, et al, 2020, Salubrious effects of a vermiculite–cellulose‐based bionanocomposite on oxidative stress indices and histomorphology of male Wistar rats, Andrologia, 52(1), 10.1111/and.13426
- 15.Moreira Lorrane Kelle da Silva, Turones Larissa Córdova, Campos Hericles Mesquita, Nazareth Aline Martins, Thomaz Douglas Vieira, et al, 2023, LQFM212, a piperazine derivative, exhibits potential antioxidant effect as well as ameliorates LPS-induced behavioral, inflammatory and oxidative changes, Life Sciences, 312(), 121199, 10.1016/j.lfs.2022.121199
- 16.Marin Daniela Eliza, Bulgaru Cristina Valeria, Pistol Gina Cecilia, 2022, Effect of agro-industrial by-products on inflammation and oxidative stress using an in vitro cell model, Archiva Zootechnica, 25(2), 97, 10.2478/azibna-2022-0017
- 17.Abarikwu Sunny O., Mgbudom-Okah Chidimma J., Ndufeiya-Kumasi Lauritta C., Monye Vivian E., Aruoren Oke, et al, 2024, Influence of triazines and lipopolysaccharide coexposure on inflammatory response and histopathological changes in the testis and liver of BalB/c mice, Heliyon, 10(2), e24431, 10.1016/j.heliyon.2024.e24431
