Effects of Different Extraction Methods on Antioxidant Properties and Allicin Content of Garlic
Abstract
The ever-present need for human communities to extract herbal active ingredients has necessitated many studies to be carried out in order to introduce more efficient and cost-effective extraction processes. The traditional extraction methods are very time consuming and use large volumes of solvents. The large volumes of solvents consumed in such methods not only increase costs but also pose many environmental problems. New extraction methods have been recently introduced to replace the traditional ones. These new methods reduce the volumes of required solvents, shorten the process and increase its efficiency and improve the quality of the products. In this study, the three methods of immersion, boiling, and ultrasound using water/ethanol solvents were employed for garlic extraction and compared with each other in terms of the extraction speed and time, the antioxidant property of the extract, and the quantity of the heat-sensitive active ingredient.
The results showed that the highest allicin content (0.086%) was observed in the ultrasonic aqueous extract. The largest amounts of phenolic compounds (0.311 mg gallic acid equivalent) were observed in the ultrasonic aqueous extract followed by the aqueous extract prepared after 72 h in a shaking incubator. The highest inhibition rate (50% at 5000 ppm) was that of the ultrasonic aqueous extract and the shaken aqueous extract. Other extracts achieved an inhibition rate of 50% at 8000 ppm. Therefore, ultrasonic extraction can be a good alternative to traditional extraction methods.
Author Contributions
Academic Editor: Amirhossein Ahmadi, Pharmaceutical Sciences Research Center, Faculty of pharmacy, Mazandaran University of Medical Sciences, Iran.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2020 Somayyeh Loghmanifar, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
The use of medicinal plants and traditional or empirical medicine started with the appearance of human life and civilization on earth because diseases have been around as long as humans. That is why all academic and industrial centers and the World Health Organization (WHO) have developed an extensive plan for the utilization of medicinal plants 1. One of the problems with modern medicine is the ever-increasing use of chemical drugs that have numerous consequences including the following ones:
The gradual emergence of autoimmunity that increases the need for using potent drugs
The undesirable and dangerous complications of chemical drugs, which are sometimes even more dangerous than the disease itself 2.
Natural antioxidants (polyphenols) found in plants are much more beneficial than synthetic antioxidants for human health. Therefore, research on natural antioxidants as a replacement for synthetic antioxidants is of particular importance 3.
Cooked or raw, garlic can be mixed with different foods and offers countless therapeutic benefits, especially in traditional medicine. Also known as Allium Sativum, garlic belongs to the Alliaceae family and is considered a gramineous herb with an onion compound containing several small bulbs 4, 5.
In addition to vitamins A, B, and C, garlic contains effective drugs such as volatile oils, mucilages, mineral salts, alliin, allicin, alliinase enzyme, and inulin. Garlic has antioxidant properties and exhibits therapeutic effects on some cancers 6. In addition, garlic has antimicrobial, antibacterial, antiviral, antifungal effects on cardiovascular and immune systems 7, 8.
Therapeutic effects of garlic include reduced levels of blood cholesterol, triglyceride, pressure, and platelet formation, antimicrobial, antifungal, anti-cancer effects, stimulation of the immune system, anti-inflammatory, and anti-oxidant effects, and prevention of arteriosclerosis 9. Recent scientific findings have also reported garlic to be helping with the absorption of drugs for cardiovascular and viral diseases 10.
Most medicinal and health properties of garlic are attributed to allicin-containing the thiosulfinate functional group. This compound is not naturally produced in the plant, but is a secondary product derived from the destruction of a kind of cysteine sulfide called alliin. As the most important enzyme in this plant family, alliinase reacts with alliin after the destruction of garlic plant tissues to produce allicin, a highly unstable compound, and pyruvic acid. Given that the amount of this composition is dependent on environmental factors and processing conditions, these parameters can be tailored to prevent the reduction of this valuable compound 6. Allicin and thiosulfinates trap free radicals and inhibit lipid peroxidation and platelet aggregation, stimulate fibrinolysis, and reduce the amount of blood lipids 4.
In the study of Lee et al. (2013), the anticancer activity of allicin and aqueous garlic extract (AGE) was investigated. They used high-performance liquid chromatography (HPLC), thin-layer chromatography (TLC), mass spectrometry (MS), nuclear magnetic resonance (NMR), chemical synthesis and MTT to determine allicin as an active anticancer compound and describe the effects of AGE. Confirming the HPLC results, further analysis by TLC, MS, and NMR described the active compound as allicin. The synthesized allicin was chemically used for further preparation. The results, therefore, clearly identified the active compound in AGE as allicin 11.
Increasing demand for greener alternatives and natural ingredients that do not contain toxic compounds and do not endanger environmental health as well as the high consumption of chemical solvents have drawn the attention of industries to non-toxic and reliable extraction methods. Hence, commercial extraction methods have been developed to obtain valuable products from plant materials. Such methods are largely focused on new solutions to reduce or even eliminate solvents in the extraction process, production of purer products, and their wider use in various applications 12.
In the study of Abdul Aziz et al (2010), 10 g of peeled edible portion of garlic was chopped, homogenized at1000 rpm for 1 min in 20 g of distilled water (1:2(w/w). The mixture was left to stand for 3 h before filtering with Whatman paper (no. 1). They reported that the aqueous garlic extract at a dose of 50 mg/kg body weight to be an effective dose in reducing blood pressure and anti-aggregatory effect on platelet aggregation 13.
In the study of Bakri and Douglas (2005) Fresh garlic cloves (70 g) were blended in 35 ml distilled water, centrifuged and ultrafiltered . They described the garlic extract (57.1% (w/v), containing 220 mg/ml allicin) inhibited the growth and killed most of the organisms tested. 14.
The purpose of this study is to investigate the three methods of immersion, boiling, and ultrasound using water/ethanol solvents for garlic extraction and compared with each other in terms of the extraction speed and time, the antioxidant property of the extract, and the quantity of the heat-sensitive active ingredient
Material and Methods
Materials
Garlic cubes were purchased from the local market of Rasht (Iran). The garlic was washed and peeled. Before extraction, the garlic was ground in a blender and the resulting particles were immediately used for extraction.
Chemicals, Reagents, and Instruments
2,2-Diphenyl-1-picrylhydrazyl (DPPH) radical, Folin-Ciocalteu reagent, and the standard substances including gallic acid were purchased from Sigma (Sigma Aldrich GmbH, Sternheim, Germany), and methanol and ethanol were prepared from Merck KGaA (Darmstadt, Germany). An ultrasonic bath process (purchased from Elma, Germany) was used to perform the ultrasonic-assisted extraction.
Methods
Garlic Extraction was Performed Using the Following Methods
The method proposed by Sharifi et al. (2003): water was selected as the solvent to prepare the extract. Small (500g) pieces of garlic were mixed with 1 liter of distilled water (ratio 1:2) using a blender. The mixture was put in a shaking incubator at 40°C for 72 h. Finally, the resulting solution was filtered and dried in a freeze dryer 15.
The method proposed by Pinilla et al. (2017), with some modifications: garlic cloves were washed, crushed and mixed with distilled water (ratio 1:2 w/v). The mixture was put in an ultrasonic bath at 40 kHz for 15 min and then centrifuged at 10,000 rpm and 4°C for 10 min. The resulting supernatant was collected, passed through a 0.22-µm membrane and stored in sterile glass containers at 4°C for the tests 16.
The method proposed by Pinilla et al. (2017), with some modifications: garlic cloves were washed, crushed and mixed with 50% ethanol (ratio 1:2 w/v). The mixture was placed in an ultrasonic bath at 40 kHz for 15 min and then centrifuged at 10,000 rpm and 4°C for 10 min. The resulting supernatant was collected, filtered using a 0.22-µm membrane, and stored in sterile glass containers at 4°C for the tests 16.
The Nazari method with some modifications: garlic cloves were peeled, washed, crushed and then mixed with distilled water (ratio 1:10 w/v). The mixture was boiled over a flame for half an hour. The resulting solution was filtered using Whatman Filter Paper No. 1 and dried 17.
The method proposed by Swami et al. (2008), with some modifications: cloves of garlic were peeled, washed, crushed and then mixed with distilled water (ratio 1:10 w/v). The mixture was put in an oven at 35°C for 24 h. The resulting solution was filtered using Whatman Filter Paper No. 1 and dried 18.
The method proposed by Swami et al. (2008), with some modifications: garlic cloves were peeled, washed and crushed and then mixed with 50% ethanol (ratio1:10 w/v). The mixture was put in an oven at 35°C for 24 h. The resulting solution was filtered using Whatman Filter Paper No. 1 and dried 18.
Determination of total Phenolic Content
The content of total phenolic compounds in the garlic extracts was determined using the Folin–Ciocalteu reagent 19. Absorbance was measured at 765 nm using a UV-VIS spectrophotometer (Shimadzu, Japan). The content of phenolic compounds was expressed as gallic acid equivalents (GAE) per dry weight of extract. All experiments were performed in three replicates.
DPPH Test
The free-radical scavenging capacity of garlic extracts was determined as described by Fenfang et al. (2017). Different amounts of the extract was mixed with methanol (95%) and DPPH in order to obtain different final concentrations of the extract. After 60 min at room temperature and in darkness, the absorbance was measured at 517 nm using a UV-VIS spectrophotometer (Shimadzu, Japan). All experiments were performed in threereplicates. Radical scavenging capacity (%RSC) was calculated from the following equation 20:
 ….(Eq. 1)
….(Eq. 1)
IC50 is a parameter commonly used to compare the free radical scavenging activity of different extracts. IC50 refers to the half-maximal inhibitory concentration of the extract against the free radical in the reaction medium 21. The amount of IC50 Obtained using Graph Pad Prism8 software.
Quantitative Determination of Allicin in the Optimum extract
The quantity of allicin in the optimum extract was measured by high-performance liquid chromatography (HPLC) (Agilent, 1200) based on the method described by British Pharmacopoeia (2015). The internal standard was used of butyl parahydroxy benzoate and column size was 0.25 m and 4 mm diameter. The mobile phase consisted of mixing 60% methanol and 40% anhydrous formic acid solution (v/ v) at a flow rate of 0.7 ml/ min and detection done with Spectrophotometer at 254 nm 22.
 ….(Eq. 2)
….(Eq. 2)
S1: area of the peak corresponding to allicin
S2: area of the peak corresponding to butylparahydroxybenzoate
C1: sample concentration
C2: internal standard concentration
Vis: internal standard volume
Vs: sample volume
Vt: total volume
Identification of the Compounds in Optimum Garlic Extract by Using gas Chromatography-mass Spectrometry (GC-MS).
This was done based on the criteria in chromatography (retention time) and spectrometry (mass spectral interpretation, comparison with library information and standard compounds). Chromatographic and spectroscopic data were collected using a gas chromatograph (Thermo Scientific Trace OQ301) attached to a DSQ mass spectrometer (electron impact ionization, eV 70; Thermo Scientific). The length of DB-5MS (J & W Scientific) column was 30 m, 0.52 mm internal diameter, with 5% phenyl polyimethylsiloxane static phase, or 0.25 μm thickness, was designed as follows:Temperature of 50 °C (1 min), temperature rise at 10 °C/ min to 280 °C and stop for 15 min. Temperature of injection area and transmission line were set to 250 and 280 °C, respectively. Helium (99.99%) as carrier gas with a pressure of 155 KPa and a rate of 27 cm/ min (constant current 1 ml/ min). Mass spectrometry and chromatograms were adjusted by mass scanning method in the range of 45-500 mlz at 5.1 scan/ sec 23.
The Statistical Analysis Method
SPSS 22 software was used for data analysis and Excel 2007 software was used for drawing charts. IC50 values were obtained using Graph Pad Prism8 software. All samples were tested in three replications.
Results and Discussion
The Results of Antioxidants Properties
(Table 1) shows the results related to total phenolic compounds based on mg gallic acid equivalent/ g dry weight of each extract. The results showed that phenolic compounds retention was affected by the extraction method and the type of solvent. The largest amounts of phenolic compounds were observed in the ultrasonic aqueous extract followed by the aqueous extract prepared after 72 h in a shaking incubator. There was a significant difference between these two extracts and the other extracts in this regard (p<0.05).
Table 1. Total phenol content by mg gallic acid equivalent / g dry weight of each extract| Sample | Ultra.aq. | Shaker aq. | Ultra. et. | oven et. | oven aq. | Boiled aq. |
| Total phenol | 0.311±0.05a | 0.295±0.04b | 0.269±0.15c | 0.216±0.05d | 0.191±0.06e | 0.112±0.2f |
The results regarding the inhibition rate of the free radical (DPPH) by the prepared extracts are shown in Figure 1. They indicated that the inhibition rate was affected by the extraction method and the type of solvent. The highest inhibition rate (50% at 5000 ppm) was that of the ultrasonic aqueous extract and the shaken aqueous extract. Other extracts achieved an inhibition rate of 50% at 8000 ppm (p<0.05).
Figure 1.Inhibition rates of the free radical (DPPH) by the different garlic extracts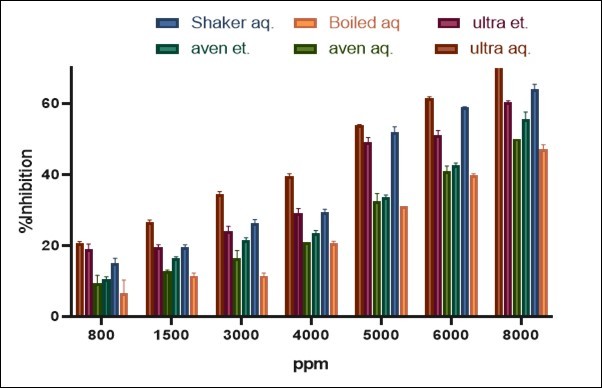
The high rate of DPPH inhibition in this study is due to the higher quantities of phenolic and tocopherol compounds. The inhibitory potency of the different extracts largely depends on the numbers and positions of the hydroxyl groups and on the molecular weights of the phenolic compounds 24. Like previous research, the results of this study showed that the extracts containing more phenolic compounds exhibited greater inhibitory effects 25. It is noteworthy that phenolic compounds act more efficiently as hydrogen donors and, therefore, serve as effective antioxidants. In addition, the inhibitory effects of phenolic compounds on DPPH increase with increases in their concentrations or hydroxylation degrees 26.
IC50 is a parameter commonly used to compare the free radical scavenging activity of different extracts. IC50 refers to the half-maximal inhibitory concentration of the extract against the free radical (DPPH in the present research) in the reaction medium. Therefore, lower IC50 values indicate higher free radical scavenging activity. Table 2 presents the IC50 values of the extracts prepared in this study. The results demonstrated that the highest and the lowest IC50 were those of the ultrasonic aqueous extract (4,376 µg/ml) and the boiled aqueous extract (8,540 µg/ml), respectively. The amounts of phenolic compounds are associated with antioxidant properties: the antioxidant property of extracts improves with increases in their content of phenolic compounds. This can justify the lower IC50 values of all boiled extracts that Table 2 shows 21.
Table 2. IC50 of garlic extracts| Sample | Ultra. aq. | Shaker aq. | Oven et. | Oven aq. | Boiled aq. |
| IC50 | 4,376a | 5,392b | 7,890c | 8,409d | 8,540d |
| R-squared | 0.91 | 0.88 | 0.91 | 0.92 | 0.93 |
The results showed that the aqueous extract outperformed the others in free radical inhibition. Moreover, there were significant differences between aqueous and ethanol extracts at all concentrations, and increased concentrations of phenolic compounds directly increased the ability of the different extracts to inhibit the activity of the free radical. Due to the increasing number of hydroxyl groups existing in the reaction medium, higher concentrations of phenolic compounds are more likely to increase the possibility of donating hydrogen to the free radicals, following which the inhibitory effects of extracts improve 27. The inhibitory potency of different extracts largely depends on the number and position of hydroxyl groups and the molecular weight of phenolic compounds. Hydroxyl groups are more available in phenolic compounds with lower molecular weights 24.
Zou et al. (2008) found that the ultrasonic extraction of ginseng saponins was about three times faster than the traditional extraction method 28. Ultrasound is considered an appropriate method for extracting biologically active compounds from Salvia officinalis and Hibiscus tiliaceus, antioxidants from rosemary 29. Compared to traditional methods in water or ethanol, the ultrasound method can increase the efficiency of extracting fennel, hops, parsley, and mint by 34%, 12%, 3%, and 7%, respectively 12. Variations in extraction efficiency are mainly due to the specific structures of the products. Albu et al. (2004) investigated the effects of different solvents and the ultrasound method on the extraction of carnosic acid from rosemary and reported that the traditional extraction method using ethanol was significantly less efficient than extraction with ethyl acetate and butanone. In addition, the application of ultrasound improved the relative efficiency of ethanol compared to the other two solvents. Therefore, the use of ultrasound may reduce the dependence on a specific solvent and makes it possible to apply alternative solvents that are more appropriate with respect to economic, environmental, health, and safety considerations 30.
The Results of the Quantitative Determination of Allicin Content
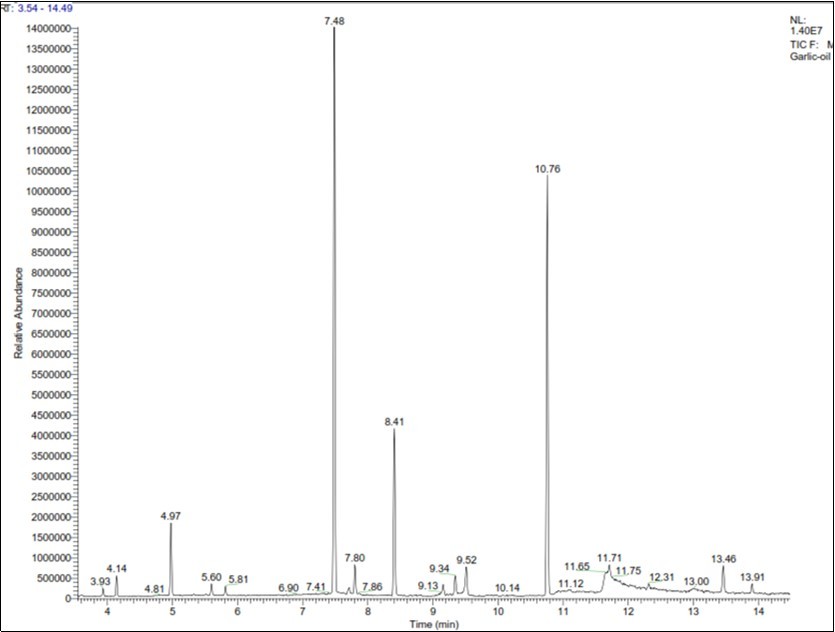
HPLC chromatograms show the results of regarding the allicin contents of the garlic extracts prepared in this study. Allicin content was calculated based on the area under the peak and Equation 2.
As shown in Table 3, the highest allicin content (0.086%) was observed in the ultrasonic aqueous extract. The allicin content significantly decreased in garlic extracts prepared in the oven, probably due to the temperature conditions. The lowest allicin content (0.009%) was that of the boiled aqueous extract. This can corroborate the decline in allicin content at high temperatures or in long exposure to heat. In fact, heat reduces the active ingredients or the enzymes effective in their production. In addition, processes like cooking soften the cell wall, facilitating the extraction of carotenoids, cause their release into the water and reduce their content in tissues 31, 32. Ilic et al. (2011) stated that allicin was derived from its precursor alliin through the activity of alliinase. Although allicin is a molecule of low polarity, it is often extracted by using polar solvents such as water at room pressure (0.1 MPa) because allicin is very unstable in non-polar organic solvents. This confirms the results of the present study 33.
Table 3. Allicin content in the different garlic extracts| Number | Sample | Area under the peak (sample) | Area under the peak (internal standard) | Allicin content (%) |
| 1 | Ultra. Aq. | 219.075 | 1158 | 0.086a |
| 2 | Shaker aq. | 72.42 | 1110 | 0.029b |
| 3 | Ultra. et. | 32.42 | 1110 | 0.013c |
| 4 | oven aq. | 31.62 | 1144 | 0.012c |
| 5 | oven et. | 27.11 | 1153 | 0.010c |
| 6 | Boiled aq. | 22.24 | 1111 | 0.009d |
The Results of the quantities of Compounds Present in the Optimum Extract, Measured by GC-MS
The quantities of compounds present in the ultrasonic aqueous extract, measured by GC-MS according to the retention time (chromatogram in Figure 2), are listed in Table 4. The two main compounds in this garlic extract were diallyl disulfide (34.87%) and dipropyl trisulfide (25.88%). Pyrogallol (13.38%) and methyl propyl trisulfide (11.36%) were among the other compounds found in the ultrasonic aqueous extract (Table 4, Figure 2). The amounts of volatile compounds in garlic extract depend on various factors including genetic factors (type and species of garlic) and agricultural factors (planting, caring for the crop and harvesting). For example, the diallyl disulfide contents of Cameroonian, Chinese, Moroccan, French and Mexican garlic are 37.3, 35, 23.2, 21.8 and 17.2%, respectively 34 45.
Table 4. The amount of compounds in ultrasonic aqueous extract| Num. | Title | RT (min) | Result (%) |
| 1 | 1,3-Dithiane | 4.97 | 4.37 |
| 2 | Dimethyl trisulfide | 5.81 | 0.56 |
| 3 | Diallyl disulfide | 7.48 | 34.78 |
| 4 | Diallyl disulfide (isomer) | 7.8 | 2.71 |
| 5 | Trisulfide, methyl 2-propenyl | 8.41 | 11.36 |
| 6 | 3-Vinyl-1,2-dithiocyclohex-4-ene | 9.16 | 0.85 |
| 7 | n-Amyl sec-butyl disulfide | 9.34 | 1.23 |
| 8 | 3-Vinyl-1,2-dithiocyclohex-5-ene | 9.52 | 2.31 |
| 9 | Trisulfide, di-2-propenyl | 10.76 | 25.88 |
| 10 | Pyrogallol | 11.71 | 13.38 |
| 11 | Hexathiane | 13.46 | 1.99 |
| 12 | Diallyl tetrasulfide | 13.91 | 0.59 |
Conclusion
In this study, the three methods of immersion, boiling, and ultrasound using water/ethanol solvents were employed for garlic extraction and were compared with each other in terms of extraction speed and time, antioxidant property and amount of heat-sensitive active ingredient. The results showed that the ultrasonic aqueous extract exhibited the highest antioxidant activity and allicin content. Ultrasonic extraction is a simple technique that can be a good alternative to traditional extraction methods. Increased the extraction speed and efficiency is one of the main advantages of the ultrasound method. Moreover, the ultrasound technique can reduce the operating temperature and allow extraction of heat-sensitive compounds.
References
- 1.Khodai M. (2014) . Antibacterial Effects of Garlic and Rosemary Essential Oil on Some Main Pathogens of Mastitis in Dairy Cattle. Cell and Tissue Journal 5, 88-79.
- 2.Binsi P K, Nayak N, Sarkar P C, Jeyakumari A, Ashraf Muhamed et al. (2017) Structural and oxidative stabilization of spray dried fish oil microencapsulates with gum arabic and sage polyphenols: Characterization and release kinetics. Food Chemistry. 219, 158-168.
- 3.Szychowski K, Rybczynska-Tkaczyk K, Tobiasz J, Stawasz Yelnytska-, Pomianek V et al.. J.(2018). Biological and anticancer properties of Inonotus obliquus extracts.Process Biochemistry 73, 180-187.
- 4.MozaffariNejad A, Shabani Sh, Bayat M, Hosseini S E. (2014) . Antibacterial Effect of Garlic Aqueous Extract on Staphylococcus aureus in Hamburger.JundishapurJMicrobiol 7(11), 131-134.
- 5.Loghmanifar S, nasiraei Roozbeh, Nouri L, Jafarian H R, S. (2019) Evaluation of nutritional value of garlic and its application in health. 4th International Congress on engineering, technology & applied sciences,New Zealand-Auckland .
- 6.Wang Y, Jia J, Shao J, Shu X, Ren X et al. (2018) Preservative effects of allicin microcapsules on daily foods. LWT -Food Science and Technology 98, 225-230.
- 7.Khoshtinat Kh, Barzegar M, Sahari M A, Hamidi Z. (2017) Encapsulation of Iranian Garlic Oil with β-cyclodextrin: Optimization and its Characterization. , J. Agr. Sci. Tech 19, 97-111.
- 8.Piletti R, Zanetti M, Jung G, Muneron de Mello, Dalcanton J et al. (2019) Microencapsulation of garlic oil by β-cyclodextrin as a thermal protection method for antibacterial action. , Materials Science & Engineering C 94, 139-149.
- 9.Balasubramani P, Viswanathan R, Vairamani M. (2013) Response surface optimisation of process variables for microencapsulation of garlic (Allium sativum L.) oleoresin by spray drying, biosystems engineering. 114, 205-213.
- 10.Cekovska S. (2010) . Extracts from the History and Medical Properties of Garlic. Pharm. Rev 4, 106-110.
- 11.Lee J, Gupta Sh, Huang J, Jayathilaka L, Lee B. (2013) HPLC–MTT assay: Anticancer activity of aqueous garlic extract is from allicin. Analytical Biochemistry. 436, 187-189.
- 12.Vinatoru M. (2001) An overview of the ultrasonically assisted extraction of bioactive principles from herbs. , Ultrasonics Sonochemistry 8, 303-313.
- 13.Aziz Abdul, Fung Tan H, Peh Y, Yam K, M. (2010) Direct effect of khat and garlic extracts on blood lipids contents: Preliminary in vitro study. , Obesity Research & Clinical Practice 4, 247-252.
- 14.I M Bakri, Douglas CWI. (2005) Inhibitory effect of garlic extract on oral bacteria. , Archives of Oral Biology 50, 645-651.
- 15.Sharifi A, Darabi R, Akbarloo N. (2003) Investigation of antihypertensive mechanism of garlic in 2K1C hypertensive rat. , Journal of Ethnopharmacology 86, 219-224.
- 16.Pinilla C M B, Norena C P Z, Brandelli A. (2017) Development and characterization of phosphatidylcholine nanovesicles, containing garlic extract, with antilisterial activity in milk. Food Chemistry. 220, 470-476.
- 17.M R Nazari, Pakzad I, Maleki A, Hematian A. (2014) Comparison of the Inhibitory Effect of Different Thirsty Extracts on Staphylococcus Aureus and Pseudomonas Aeruginosa and Helicobacter Pylori Bacteria in In vitro. , Scientific Journal Ilam Medicine 3, 22.
- 18.Swami S, Preet S, Khanuja S. (2008) . Extraction Technologies for Medicinal and Aromatic Plants.International Centre for Science and High Technology .
- 19.Petropoulos S, Fernandes A, Barros L, Ana Ciric Sokovic, Ferreira M et al. (2017) Antimicrobial and antioxidant properties of various Greek garlic genotypes. Food Chemistry. 8146(17), 31716-8.
- 20.Fenfang L, Qiao L, Shuanggen W, Zhijian T. (2017) Salting-out extraction of allicin from garlic (Allium sativum L.) based on ethanol/ammonium sulfate in laboratory and pilot scale. Food Chemistry. 217, 91-97.
- 21.Pinelo M, Fabbro Del, Marzocco P, Nunez L, M J Vicoli et al. (2005) Optimization of continuous phenol extraction from Vitis vinifera byproducts. , Food Chem 92, 109-117.
- 23.R P Adams. (2001) Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry.Allured Publishing Corporation Carol Stream. , IL
- 24.C H Jung, H M Seog, I W Choi, M W Park, H Y Cho. (2006) Antioxidant properties of various solvent extracts from wild ginseng leaves. , LWT 39, 266-274.
- 25.Maleki M, Ariaii P, Fallah H. (2015) Effects of Celery Extracts on the Oxidative Stability of Canola Oil Under Thermal Condition: Antioxidant Effect of Celery Extract on Canola Oil". Journal of Food Processing and Preservation.
- 26.Golluce M, Sahin F, Sokmen M, Ozer H, Daferera D et al. (2007) Antimicrobial and antioxidant properties of the essential oils and methanol extract from Mentha longifolia L. , ssp. longifolia. Food Chem 103, 1449-1456.
- 27.Ghaderi M, Sadeghi A, Aalami M, Ghorbani M, M H Azizi. (2011) Evaluation of anadromic activity. regenerative power and antioxidant capacity of phenolic extracts of an oak variety. , Journal of Food Technology Research 21, 1.
- 28.Zou C, Y M Du, Li Y, J H Yang, Feng T et al. (2008) Preparation of lacquer polysaccharide sulfates and their antioxidant activity in vitro. , Carbohyd. Polym 73, 322-331.
- 29.Wang L, C L Weller. (2006) Recent advances in extraction of nutraceuticals from plants. Trends Food Sci",Technol. 17, 300-312.
- 30.Albu S, Joyce E, Paniwnyk L, Lorimer P, Mason J. (2004) Potential for the use of ultrasound in the extraction of antioxidants from Rosmarinus officinalis for the food and pharmaceutical industry. , Ultrasonics Sonochemistry 11, 261-265.
- 31.Shirzad H, Taji F, Rafieian M. (2011) The Influence of Heat on Garlic Active Materials. , J. Armaghane-danesh 1, 16.
- 32.D B Rodriguez-Amaya. (1999) Changes in carotenoids processing and storage of foods. , Arch Latinoam Nutr 49, 38-47.
- 33.D P Ilic, V D Nikolic, L B Nikolic, M Z Stankovic, L P Stanojevic. (2011) Cakic MD. "Allicin and related compounds: Biosynthesis, synthesis and pharmacological activity". , Physics, Chemistry and Technology 9(1), 9-20.
Cited by (19)
This article has been cited by 19 scholarly works according to:
Citing Articles:
Future Foods (2025) Crossref
J. Akullo, B. Kiage-Mokua, Dorothy Nakimbugwe, Justus Kwetegyeka, J. Ng’ang’a et al. - Future Foods (2025) Semantic Scholar
Future Foods (2025) OpenAlex
Sumit W Ingole, Vaibhav P. Kakde, Maroti M. Jeurkar, D. N. O. Chachda, Saloni S Raut et al. - International journal of pharmaceutical research and applications (2025) Semantic Scholar
Life (2024) Crossref
Ștefania Dinu, Ștefania-Irina Dumitrel, Roxana Buzatu, Dorin Cristian Dinu, R. Popovici et al. - Life (2024) Semantic Scholar
Life (2024) OpenAlex
Chemical Engineering Journal (2024) Crossref
Sangsu Lee, Kyusun Kim, Ho Dong Son, Hee Jeong Jeong, Sang Ho Won et al. - Chemical Engineering Journal (2024) Semantic Scholar
Chemical Engineering Journal (2024) OpenAlex
(2024) OpenAlex
Heliyon (2023) Crossref
Heliyon (2023) OpenAlex
J. Akullo, B. Kiage-Mokua, D. Nakimbugwe, J. Ng’ang’a, J. Kinyuru - Heliyon (2023) Semantic Scholar
Advances in Animal and Veterinary Sciences (2022) OpenAlex
International Journal of Veterinary Science (2022) OpenAlex
Frontiers in Nutrition (2022) Crossref
Frontiers in Nutrition (2022) OpenAlex
Christiana Oluwatoyin Ajanaku, O. T. Ademosun, Prudence Osahenomanse Atohengbe, S. Ajayi, Y. Obafemi et al. - Frontiers in Nutrition (2022) Semantic Scholar
Antioxidants (2022) Crossref
Antioxidants (2022) OpenAlex
M. Bar, Urszula E Binduga, K. A. Szychowski - Antioxidants (2022) Semantic Scholar
Somayyeh Loghmanifar, Leila Roozbeh Nasiraie, Hamidreza Nouri, Sara Jafarian - Scientia Iranica. International Journal of Science and Technology (2022) Semantic Scholar
International Journal of Veterinary Science (2022) OpenAlex
Somayyeh Loghmanifar, L. R. Nasiraie, H. Nouri, Sara Jafarian - (2020) Semantic Scholar