Delayed Vision Loss Post Assault-Induced Zygomatic Fracture Repair in a Patient with an Ipsilateral Anterior Clinoid Process Pneumatization, A Case Report
Abstract
A 27-year-old male presented one week after surgical repair of his fractured left zygomatic arch, 5 weeks post-assault, complaining of persistent blurred vision in the left eye and worsening migraine headaches. Magnetic resonance images (MRI) revealed no intra-orbital pathologies or optic neuropathy but demonstrated a clearly delineated air-filled space of the left anterior clinoid process (ACP). No vision threatening pathologies were identified and surgical treatment was not recommended. His blurred vision remained at 6-month follow up. We report a case of delayed vision loss and headache post zygomatic fracture repair surgery in patient with a confirmed ipsilateral ACP pneumatization.
Author Contributions
Academic Editor: Shuai Li, University of Cambridge.UK
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2023 Elleny M. Gutierrez, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have no intellectual conflicts of interest to declare.
Citation:
Introduction
Anatomical variations of the orbit can have a direct impact on surgical approaches and outcomes. Anatomic configurations that predispose the optic nerve to injury include type 2 (nerve course adjacent to the sphenoid sinus causing an indentation of the sinus wall, without contacting any posterior ethmoid cell) or type 3 (nerves course through the sphenoid sinus), bone dehiscence over the optic nerve, and anterior clinoid process pneumatization (ACPP) with and without ipsilateral optic nerve protrusion.1, 2, 3, 4 The anterior clinoid process (ACP) is a posterior and medial continuation of the lesser sphenoid wing, connecting to the sphenoid bone body via superior and inferior roots.5, 6 The ACP is on the superolateral aspect of the sphenoid sinus lying between the internal carotid artery and the optic nerve. Pneumatization refers to the presence of an air-filled cavity of variable size adjacent to the ACP. Pneumatization occurs when a paranasal sinus, often the sphenoid or ethmoid sinus, extends into the anterior clinoid process via the inferior or anterior root otherwise known as the optic strut. Anatomic variations in size and patterns of ACPP are distinguished by cranial computed tomography (CT) imaging.5, 6, 7, 8, 9, 10, 11The reported incidence of unilateral and bilateral ACPP varies between populations with a worldwide range from 6–35%.5, 8, 9, 10, 11, 12ACPP develops in males and females,10,11 was reported to be less in females <20 years of age,8and does not progress.11ACPP is not associated with any predictors that might be recognized preoperatively12 and has no reported pathologic consequences despite its proximity to the internal carotid artery, the ophthalmic artery, and the optic nerve. Awareness of the variations in pneumatization can prevent destruction of the mucous membrane and facilitate reconstruction.5
We report an uncommon case of delayed onset blurred vision and migraine headache post-surgical repair of an assault-induced left zygomatic arch fracture in a patient with an ipsilateral ACPP.
Case Report
A 27-year-old male with a past medical history of diabetes mellitus was referred to the ophthalmology clinic complaining of persistent blurry vision in the left eye and worsening migraine headaches. The patient’s past ophthalmic history was unremarkable, except for refractive error corrected with glasses. The patient’s immediate past medical history included uneventful surgical repair of the closed left zygomatic arch fracture 7 days earlier (5 weeks after being assaulted) under general anesthesia by the Oral and Maxillofacial Surgery Service at Louisiana State University Health-Shreveport. The repair was accomplished via an intraoral Keen approach to the zygomatic maxillary buttress. Dissection of the arch with a #9 elevator was done to encounter the fracture site on the arch. A Seldin retractor and row elevator were used to mobilize the fracture into alignment. The wound was then irrigated and closed with 3-0 Vicryl. Postoperative medications included Amoxicillin, Ibuprofen, Hydrocodone, and Peridex. The external ophthalmic exam revealed no involvement of the intra-orbital space or extraocular muscles and no restriction of extraocular muscles. The patient’s visual acuity by projected Snellen chart was 20/20 in the right eye and 20/400 in the left eye. His pupils were reacted to light equally with no relative afferent pupillary defect (RAPD). The anterior segment exams and intraocular pressures by applanation (12 mmHg OD and 15 mmHg OS) were within normal limits. Posterior segment exams using the indirect ophthalmoscope revealed no signs of optic disc pallor or edema and no macular/retinal pathology (i.e., no diabetic retinal disease detected). Optical coherence tomography (OCT)of the retinal nerve fiber layer showed symmetric thinning of the retinal nerve fiber layer both eyes superiorly (average thickness values 82 microns OD and 78 microns OS). Humphrey visual field 24-2 testing demonstrated non-specific spotty field defects in the right eye and a diffuse visual field depression in the left eye (no specific vertical or horizontal field cut).
Based on the patient’s surgical history, ophthalmic exam, OCT, and 24-2 test results, brain and orbit MRI with and without contrast were ordered to identify lesions along the visual pathway, notably the optic nerve tract, chiasm, or occipital lobe. The MRI revealed no intra-orbital pathology and no abnormal enhancement of the optic nerve/chiasm/radiations or brain parenchyma. Of note, axial post-gadolinium T1-weighted MRI (Figure 1 A) demonstrated an area of hypointensity in the left ACP that measured 1.46 cm x 1.31 cm consistent with the presence of air-filled space involving about 50% of the left ACP. The coronal view of his gadolinium T1-weighted MRI (Figure 1B) clearly delineated the air-filled space with no obvious hematoma or traumatic optic nerve lesions noted. The axial T2-weighted FIESTA-C (modification of the basic FIESTA/True FISP sequence) MRI (Figure 1C) showed hypointensity in the left ACP. There was no discernible optic canal stenosis, change in optic nerve course, or optic nerve dehiscence or protrusion associated with the ACPP. The patient was referred to neurosurgery for further evaluation. His head and orbit CT scans revealed no bony fragment or hematoma affecting structures in the visual pathway. No surgical intervention was entertained, as the risks significantly outweighed any perceived benefit. At 6 months follow-up, the patient had no improvement of blurred vision in his left eye.
Figure 1.(A) Axial post-gadolinium T1-weighted MRI demonstrating the T1 hypointensity in the left anterior clinoid process (yellow arrow). (B) Coronal post gadolinium T1-weighted MRI with two arrows outlining a clearly delineated air-filled space without a hematoma or traumatic lesion to optic nerve just superior to the tip of blue arrow. (C) Axial T2-weighted FIESTA-C MRI showing T2 hypointensity in the left anterior clinoid process (yellow arrow).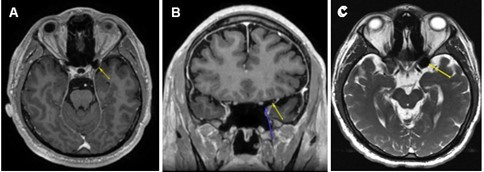
Discussion
To our knowledge this is the first case of delayed monocular vision loss post-surgery in a patient with a confirmed ipsilateral ACPP. Buckley reported a similar case who developed monocular blindness 9 days after being assaulted with a baseball bat: 7 days after zygomaticomaxillary complex fracture repair.13 Thus, our case is the second assault case of delayed visual loss post-zygomatic fracture repair surgery. No objective reason was detected for the delayed vision loss by his initial head trauma evaluation or after the open reduction of the zygomatic arch. There were no bony changes to sphenoid or ethmoid bones or soft tissue. The vision loss did not resolve with reduced swelling. Ocular complications following maxillofacial fracture treatment include blurred vision (2.4%), diplopia (3.2%) and blindness (0.84%).14 The overall risk for vision loss due to hemorrhage, optic nerve ischemia, optic nerve trauma, or malpositioned implant post orbital surgery is 0.84-2.08%.15,16Meta-analysis of surgical outcomes post orbital surgery in the ACPP patient subgroup is currently unavailable.
Patients who had sustained midfacial trauma due to assault or fall are at risk for vision loss due to impaired convergence and accommodation related to the severity of both the impact and the associated closed head trauma.17 Our patient’s blurred vision was manifested at near and distance and occurred post-surgery. Also, pneumatization of the optic sheath after severe head trauma post assault can cause optic nerve injury and reduced vision. The likely mechanism of optic nerve pneumatization is indirect, occurring as an extension of a pneumocephalus into the subarachnoid space that then surrounds the optic nerve.18The optic nerve sheath did not appear surrounded by air or have other irregularity and optic nerve protrusion or dehiscence was not evident by MRI or CT.
Mucocele (fluid-filled cystic lesion) of the ACP is a rare cause of optic neuropathy but can develop spontaneously in a pneumatized ACP because of inflammation. Mucocele of the ACP usually presents as an acute, rapidly progressive, and painless monocular vision loss but additional symptoms may include headache, diplopia and limited mobility in the affected eye.19,20Surgical mucocele decompression usually results in complete recovery.21A mucocele was not evident in our patient’s MRI or CT scans.
Posterior ischemic optic neuropathy (PION), due to ischemic insult to the visual tract or visual cortex, is an uncommon cause of vision loss after trauma or surgery.22,23 The vision loss is painless, occurs immediately after recovery or within several days of the surgery. Preexisting factors that have been associated with perioperative PION are male sex, obesity, obstructive sleep apnea, and amiodarone (phosphodiesterase-5 inhibitor).22A normal funduscopic examination is demonstrated at the onset of their symptoms, but optic disc atrophy often develops 4-8 weeks later. PION patients typically present with bilateral, painless vision loss that occurs immediately after recovery or within several days. A normal funduscopic examination is demonstrated at the onset of their symptoms, but optic disc atrophy often develops 4-8 weeks later. Moreover, in the case of unilateral disease, the patient presents with an ipsilateral RAPD.24PION is often a diagnosis of exclusion. No treatment has proven to be effective. The prognosis for visual recovery is generally poor.25Our patient’s monocular worsening vision, as well as his lack of RAPD and optic disc atrophy decreased our suspicion of PION as the etiology for the vision loss.
ACPP occurs in a significant proportion of the world population but has not been reported to be a significantly health risk. In our case, pathology consistent with the functional vision loss was not detected, leaving the possibility that the post-surgery structural abnormality of ACPP as the co-contributing factor. Thus, treatment for this patient’s vision loss would consist of eliminating the one abnormality seen on examination/imaging: the pneumatization of the ACP. ACPP is a concern during pre-operative evalation for an anterior clinoidectomy requiring specific planning for anatomic variants, since tearing of these paranasal sinuses or mucosa can occur causing pneumocephalus or liquorrhea that may damage nearby structures.6, 16,18In this case, post operation complications and risk of optic nerve injury outweighed the probability that surgical intervention would return or improve this patient’s vision. Delayed vision loss post-surgery in patients with trauma and without a clear underlying etiology remains difficult from a diagnostic and treatment standpoint.
Conclusion
The current case of delayed vision loss after surgical repair of assault-induced zygomatic fracture supports evaluation for an ACPP etiology in facial trauma patients with an unknown delayed vision deficit following uneventful orbital surgery.
Abbreviations
ACP, anterior clinoid process; ACPP, anterior clinoid process pneumatization; CT, computed tomography; FIESTA-C, fast imaging employing steady-state acquisition-constructive; MRI, magnetic resonance images; OCT, optical coherence tomography; PION, posterior ischemic optic neuropathy; RAPD, relative afferent pupillary defect.
Ethics approval and consent to publication
The study was approved by the LSU HRPP/Internal Review Board as non-research patent care (No.00000918). The investigation was performed according to the ethical standards in the Declaration of Helsinki. The patient has given written informed consent (available upon request) to publish his case and images.
Availability of data
All pertinent data are presented in the manuscript.
Financial disclosure
The authors have no financial disclosures to declare.
Authors Contribution Statement
All authors participated in drafting the article or revising it critically for important intellectual content, and all authors approved the last version to be published. Dr. Byrd and Dr. Brinkley had full access to all the data in the clinical picture and take responsibility for the integrity of the data and the accuracy of the data analysis. Study conception and design: WAB and JJB. Acquisition of data: EMG, BAS, JJB, WAB. Analysis and interpretation of data: WAB, JJB, EMG, BAS, MPL. Writing of manuscript: WAB, MPL, JJB.
Acknowledgements
The authors recognize Julio Vega (MS3) for his contributions in data acquisition. The research was supported in part by an Allergan unrestricted grant to support Resident Education and Research in the Department of Ophthalmology, Louisiana State University Health Sciences Center, Shreveport, LA.
References
- 1.DeLano M C, Fun F Y, Zinreich S J. (1996) Relationship of the optic nerve to the posterior paranasal sinuses: a CT anatomic study.AJNR. , Am J Neuroradiol.17: 669-675.
- 2.Kim J, Park S W, Choi J, Jeong W, Kim R. (2018) Effects of infraorbital nerve's anatomical course on the fracture pattern of the orbital floor. , J Plast Reconstr Aesthet Surg 71, 490-495.
- 3.Ugradar S, Goldberg R, Rootman D. (2019) Anatomic variation of the entrance of the optic canal into the orbit. , Orbit 38, 305-307.
- 4.Raseman J, Guryildirim M, Beer-Furlan A, Jhaveri M, Tajudeen B A. (2020) Preoperative computed tomography imaging of the sphenoid sinus: striving towards safe transsphenoidal surgery. , J Neurol Surg B Skull Base 81, 251-262.
- 5.Mikami T, Minamida Y, Koyanagi I, Baba T, Houkin K. (2007) Anatomical variations in pneumatization of the anterior clinoid process. , J Neurosurg 106, 170-174.
- 6.Ota N, Tanikawa R, Miyazaki T, Miyata S, Oda J.(2015).Surgical microanatomy of the anterior clinoid process for paraclinoid aneurysm surgery and efficient modification of extradural anterior clinoidectomy.World. , Neurosurg 83, 635-643.
- 7. (1999) Arslan H, Aydinlioğlu A, Bozkurt M, Egeli EA. Anatomic variations of the paranasal sinuses: CT examination for endoscopic sinus surgery. Auris Nasus Larynx.. 26, 39-48.
- 8.MDS Costa, SBF Oliveira, Araujo P D, Rodrigues T P, Abdala N. (2016) Anatomical variations of the anterior clinoid process: A study of 597 skull base computerized tomography scans.Oper Neurosurg (Hagerstown).12: 289-297.doi 10.1227/NEU.0000000000001138.
- 9.Engin Ӧ, GFJPM Adriaensen, FWA Hoefnagels, Saeed P. (2021) A systematic review of the surgical anatomy of the orbital apex. , Surg Radiol Anat 43, 169-178.
- 10.Abuzayed B, Tanriover N, Biceroglu H, Yuksel O, Tanriover O. (2010) Pneumatization degree of the anterior clinoid process: a new classification. , Neurosurg Rev 33, 367-73.
- 11.Burulday V, Muluk N B, Akgül M H, Kaya A, Öğden M. (2016) Presence and types of anterior clinoid process pneumatization, evaluated by multidetector computerized tomography. , Clin Invest Med 39, 105-10.
- 12.Szmuda T, Sloniewski P, Baczalska A, Cabala M, Czapski B. (2013) The pneumatisation of anterior clinoid process is not associated with any predictors that might be recognized preoperatively. , Folia Morphol (Warsz) 72, 100-106.
- 13.Buckley S B, McAnear J T, Dolwick M F, Aragon S B. (1985) Monocular blindness developing 7 days after repair of zygomaticomaxillary complex fracture. A clinical report. Oral Surg Oral Med Oral Pathol. 60, 25-28.
- 14.Motamedi M H, Dadgar E, Ebrahimi A, Shirani G, Haghighat A. (2014) Pattern of maxillofacial fractures: a 5-year analysis of 8,818 patients. , J Trauma Acute Care Surg 77, 630-634.
- 15.Jacobs S M, McInnis C P, Kapeles M, Chang S H. (2018) Incidence, risk factors, and management of blindness after orbital surgery. , Ophthalmol 125, 1100-1108.
- 16.Kansakar P, Sundar G. (2020) Vision loss associated with orbital surgery - a major review. , Orbit 39, 197-208.
- 17.al-Qurainy I A. (1995) Convergence insufficiency and failure of accommodation following midfacial trauma. , Br J Oral Maxillofac Surg 33, 71-75.
- 18.Cetinkaya E A, Koc K, Kucuk M F, Koc P, Muluk N B. (2017) Calculation of an optic nerve injury risk profile before sphenoid sinus surgery. , J Craniofac Surg 28, 75-78.
- 19.Schwaighofer B W, Sobel D F, Klein M V, Zyroff J, Hesselink J R. (1989) Mucocele of the anterior clinoid process:. , CT and MR findings. J Comput Assist Tomogr 13, 501-3.
- 20.Abozed M, Alsulaiti G, Almannaei F, Raza A, A El Beltagi. (2017) Anterior clinoid mucocele causing optic neuropathy: A case report and review of literature. , eNeurologicalSci 7, 57-59.
- 21.Zhang S, Li Z, Ye H, Zhao H, Wu Q. (2020) A case report of unrecovered monocular vision loss after anterior clinoid mucocele resection. , Am J Ophthalmol Case Rep 20, 100980-10.
- 22.Eid J J, Cronin B C, Seman S. (2018) An unforeseeable complication; Posterior ischemic optic neuropathy after penetrating injury to the heart. , Bull Emerg Trauma 6, 178-180.
- 23.Lee A G. (1995) Reversible loss of vision due to posterior ischemic optic neuropathy. , Can J Ophthalmol 30(6), 327-9.
Cited by (4)
This article has been cited by 4 scholarly works according to:
Citing Articles:
