Changes in Adult Rats’ Testis structure Induced by Hypothyroidism and Alleviating Role of L-Carnitine
Abstract
Background
Hypothyroidism is a metabolic disorder affecting the functions of many tissues in the body including the testis. Testis is rich in the polyunsaturated fatty acids content and lacks strong intrinsic antioxidant system making it prone to such oxidative stress. L-carnitine (LC) regulates long chain fatty acids metabolism; and is considered a valuable antioxidant factor.
Aim
It was to evaluate the effect of hypothyroidism induced by propylthiouracil (PTU) on rats’ testes and the possible protective role of LC.
Methods
Forty-eight adult male albino rats were used in this work. The animals were divided into three groups with sixteen animals in each. Group 1 (Control): Animals were kept without medications. Group 2 (PTU-treated): was subjected to administration of PTU; while group 3 (PTU and LC) received both PTU and LC. By the end of the experiment “30 days”, blood samples were taken for hormonal assay; then animals were anaesthetized and sacrificed. Specimens were homogenized for biochemical analysis; epididymal content of each rat was obtained immediately for semen analysis. Testes’ specimens were harvested, prepared and examined by light microscope examination.
Results
Induced hypothyroidism was noticed to cause histopathological, morphometric and biochemical changes in rat’s testes. LC protected the testicular specimens against such changes; it also improved the seminal quality and quantity as well as testicular structure and biochemistry.
Conclusion
Hypothyroidism could result in hazards to the structure of testis. Fortunately co-administration of LC might reduce such hazards.
Author Contributions
Academic Editor: Hesham N. Mustafa, Associate Professor of Anatomy, Faculty of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2019 Abdelmonem Awad Hegazy, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
The human body structure is vulnerable to histopathological changes resulting from exposure to many internal and external ‘environmental’ elements. Investigating the structure is inevitable to determine such changes and their hazards in trial to reduce or alleviate them 1. Thyroid hormones have their own receptors in various tissues; and interaction between them is concerned with regulation of cellular metabolism in many tissues such as brain, blood, heart, liver and muscle 2, 3. Testicular tissues have also receptors for thyroid hormones. These receptors are present and functioning throughout the life 4.
During normal metabolism, oxygen is utilized with production of reactive oxygen species (ROS) which are severely toxic to cells5.These ROS can oxidize the DNA, lipids and proteins with subsequent cellular death 6. Cells have their own antioxidant defense against such ROS protecting the cellular contents 7. The oxidative stress occurs when the ROS accumulation is higher than the capacity of the antioxidant defense which initiates a pathological environment that could end with cellular death 8.
Low thyroid hormone levels are accompanied by reduction in the antioxidant defense with subsequent loss of testicular defense mechanism against ROS produced by the cellular metabolism9, 10. Deficiency of thyroid hormones during early stages of development and maturation was shown to hinder the normal testicular growth and physiology. However, few studies were done to evaluate the effect of hypothyroidism on adult testes and highlight its effect on the testicular antioxidant system 9.
LC is an amino acid like vitamin used in various conditions such as renal failure, with anti-convulsive therapy, controlling body weight and male infertility 11. It has an antioxidant activity which regulates fatty acid metabolism and prevents accumulation of toxic acetyl- CoA within the cell membranes 12.The aim of this work was to evaluate the effect of hypothyroidism on testes with reference to the possible preventive role of l-carnitine using biochemical, histological and morphometric investigation.
Material and Methods
Chemicals
1. Propylthiouracil (PTU) was obtained in the form of powder, from Amoun Pharmaceutical Company, El-Obour City, Cairo, Egypt.
2. L-carnitine (LC) was obtained in the form of ampoules (1gm/ 5ml), from El Amirya Company for pharmaceuticals industries, Alexandria, Egypt
Experimental Animals
The study was performed on forty-eight healthy adult male Wistar albino rats, aged 5-7 months and weighing 180-230 gm. The animals were obtained from the animal house, Faculty of Medicine, Zagazig University. The animals were housed under controlled laboratory conditions. All experimental procedures were performed in accordance with the guidelines of the Institutional Animal Care and the norms of Ethical Committee of Faculty of Medicine; Zagazig University. The rats were divided into 3 groups as follows:
First (Control) Group
Animals were kept without any medications.
Second (PTU-Treated) Group
Rats were subjected to administration of PTU powder dissolved in drinking water at a dose of 1mg/ml/day (13).
Methods
By the end of the experiment which was 30 days, the animals were weighed; and venous blood samples were collected form the retro-orbital venous plexuses under light ether anesthesia for measurement of the thyroid hormones (T3&T4). All the animals were anaesthetized by ether inhalation; and epididymal content of each rat was obtained immediately for semen analysis. Testes were dissected out. Weights and volumes of each testis were measured. Specimens were minced and homogenized for biochemical study of the oxidative enzymes; reduced glutathione (GSH), catalase (CAT), superoxide dismutase (SOD) and malondialdehyde (MDA). Testicular specimens were obtained for histological study. The perimeter of seminiferous tubules was measured from X-100 photo; and the height of germinal epithelium was measured from X-400 photos using Digimizer 4.3.2. image analysis software for morphometrical analysis. Moreover, statistical analysis was performed; quantitative data were expressed as mean ±SD (standard deviation) and then ANOVA (F-test) test was used to calculate difference between quantitative variables in mobre than two groups in normally distributed data15.
Results
1. Rat Body Weight, Testicular Weights and Testicular Volumes
There were statistically significant differences between the studied groups regarding rat body weight at the end of experiment. On LSD (least significance difference) comparison, the difference was found between each two individual groups as there was a significant decrease in rat body weight in the PTU-treated group while the body weight in both control and PTU&LC-treated groups were increased. There was also a significant difference between these groups regarding right and left testis weights, with control group having the most significant heavier testis; and the PTU-treated group had the least testicular weight. There was also significant difference between these groups regarding right and left testis volume, with PTU-treated group showing the most significant higher testis volume and PTU&LC-treated group had the least volume (Table 1; Figure 1, Figure 2, Figure 3).
Table 1. Rat weight and testis weight and volume of studied groups.| Control group | PTU-treated group | PTU and LC-treated group | F test | p | |
| Post-sacrifice body weight (gm)Mean ±SD(Range) | 200 ±01.15(198 -202) | 190.25 ±10.75(185 - 230) | 237.31 ±2.8(230 - 240) | 237.52 | <0.001 |
| Right testis weight (gm)Mean ±SD(Range) | 1.87 ±0.05(1.8 –1.9) | 1.39 ±0.25(1.1 – 1.75) | 1.47 ±0.15(1.2 – 1.72) | 36.92 | <0.001 |
| Left testis weight (gm)Mean ±SD(Range) | 1.83 ±0.09(1.7 – 1.9) | 1.36 ±0.17(1.14 – 1.6) | 1.49 ±0.14(1.2 – 1.7) | 49.4 | <0.001 |
| Right Testis volume (ml)Mean ±SD(Range) | 2.8 ±0.87(2.4 –5.02) | 4.92 ±0.09(4.75 – 5) | 2.54 ±0.14(2.35 – 2.85) | 105.49 | <0.001 |
| Left Testis volume (ml)Mean ±SD(Range) | 2.88 ±0.89(2.41 –5.02) | 4.94 ±0.13(4.7 – 5.2) | 2.63 ±0.15(2.41 – 2.95) | 93.29 | <0.001 |
Figure 1.Bar chart showing rat body weight in studied groups
Figure 2.Bar chart showing right and left testes weight in studied groups.
Figure 3.Bar chart showing right and left testes volume in studied groups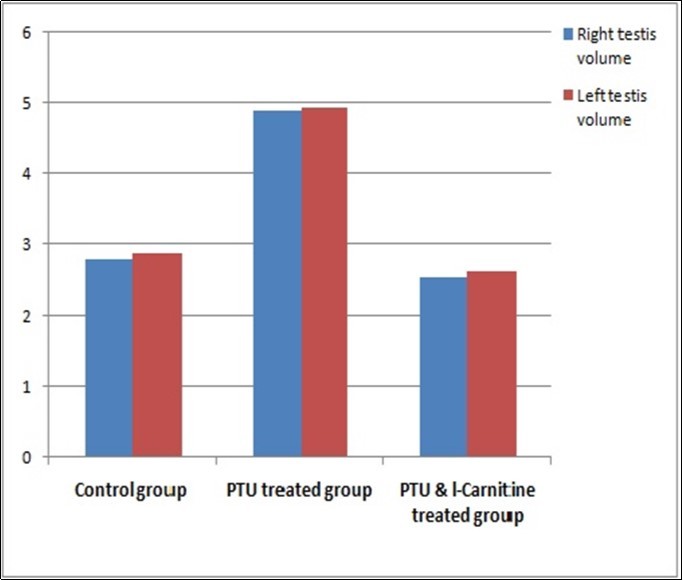
2. Thyroid hormones’ Levels
There was a statistically significant difference between the studied groups regarding free T3. On LSD comparison, the difference was present between each two individual groups as its level was significantly decreased in both PTU-treated and PTU&LC-treated groups compared with the control group. There was highly significant difference between total T4 in the different groups; on LSD comparison, the control group had the most significant higher T4 level as T4 level was significantly decreased in both PTU-treated and PTU& LC-treated groups compared with the control group (Table 2, Figure 4).
Table 2. Thyroid hormones levels in the studied groups| Control group | PTU-treated group | PTU and LC-treated group | F | p | |
| Total T3 (ng/ml)Mean ±SD(Range) | 1.59 ±0.01(1.57 -1.61) | 0.49 ±0.02(0.47-0.51) | 0.41 ±0.05(0.36 - 0.46) | 6564.9 | <0.001 |
| Total T4 (μg/ml)Mean ±SD(Range) | 5.61 ±0.01(5.59 – 5.63) | 0.62 ±0.05(0.569 – 0.667) | 0.61 ±0.05(0.565 – 0.663) | 75822 | <0.001 |
Figure 4.Bar chart showing total T3 and total T4 in studied groups.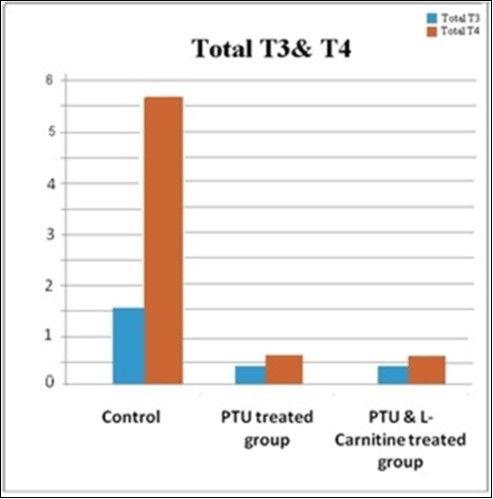
3. Semen Analysis
There was a statistically significant difference between the studied groups regarding sperm count, abnormal sperm form and sperm mobility. On LSD comparison, the difference was significant between each two individual groups as sperm counts and abnormal sperm forms percentage were significantly high in the PTU-treated and significantly decreased in the PTU&LC-treated group compared with the PTU-treated group. On the other hand, sperm motility was significantly diminished in the PTU group compared with the PTU&LC-treated group which was significantly higher (Table 3; Figure 5, Figure 6).
Table 3. Semen analysis in studied groups| Control group | PTU-treated group | PTU and LC-treated group | F | P | |
| Sperm count(million/ml)Mean ±SD(Range) | 84.06±0.85(83 -85) | 65.81 ± 3.53(61 - 70) | 81.75 ±1.69(80 - 85) | 295.75 | <0.001 |
| Abnormal sperm form (%)Mean ±SD(Range) | 8.14±0.01(7.56 –8.63) | 39± 3.45(36.17 – 41.33) | 26.3 ± 3.44(22.45 – 30.71) | 729.1 | <0.001 |
| Sperm motility (%)Mean ±SD(Range) | 84.31±0.79(83 -85) | 45.88 ± 2(43 - 48) | 59.44 ±0.51(59 - 60) | 3741.8 | <0.001 |
Figure 5.Bar chart showing sperm count in studied groups.
Figure 6.Bar chart showing abnormal sperm form and sperm mobility in studied groups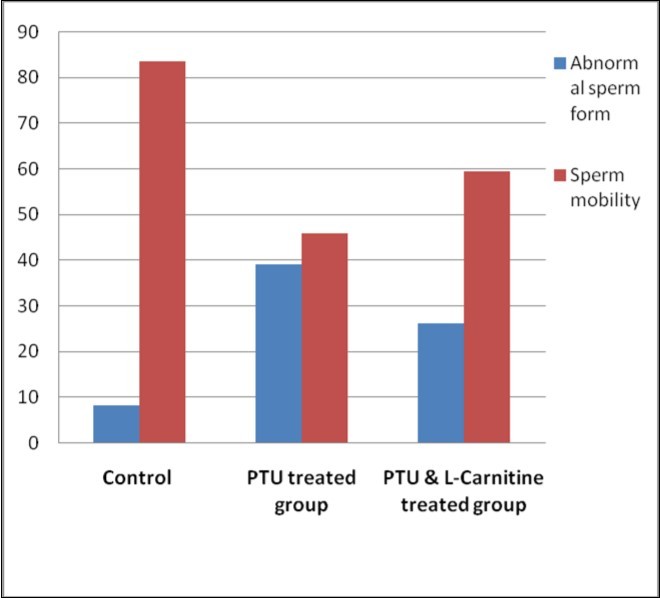
4. Oxidative Enzymes
There was statistically significant difference between the studied groups regarding GSH; on LSD comparison, GSH level was significantly diminished in the PTU-treated group compared with PTU&LC-treated groups. There was also significant difference between these groups regarding CAT and SOD; on LSD comparison the difference was significant between each two groups as their levels were significantly diminished in the PTU-treated group compared with PTU& LC-treated groups. There was significant difference between these groups regarding MDA; on LSD comparison, its level was significantly increased in the PTU-treated group compared with PTU&LC-treated groups (Table 4; Figure 7).
Table 4. Oxidative enzymes in the studied groups| Control group | PTU-treated group | PTU and LC-treated group | F | P | |
| GSH (mmol/ g)Mean ±SD(Range) | 1.29±0.004(1.28 -1.29) | 1.07 ± 0.02(1.042 – 1.086) | 1.39±0.31(1.19 – 1.92) | 12.96 | <0.001 |
| CAT(U/ g tissue)Mean ±SD(Range) | 1.72±0.01(1.71 –1.73) | 1.51 ± 0.003(1.502 – 1.511) | 1.67 ± 0.06(1.605 – 1.734) | 179.46 | <0.001 |
| MDA(mmol/ g)Mean ±SD(Range) | 1.14 ± 0.21(0.89 – 1.37) | 1.66 ±0.01(1.65 – 1.67) | 1.53 ±0.05(1.46 – 1.59) | 78.17 | <0.001 |
| SOD(U/ g tissue)Mean ±SD(Range) | 3.87±0.13(3.67 –3.99) | 2.85 ± 0.33(2.06 – 3.08) | 2.98 ± 0.65(2.3 – 3.61) | 27.63 | <0.001 |
Figure 7.Bar chart showing oxidative enzymes in studied groups
5. Light Microscopic Examination
H&E Stain
Sections of adult albino rats’ testes stained with H&E in the control group showed that multiple round seminiferous tubules with regular contour were packed within the testicular parenchyma (Figure 8A). Their lining consisted of stratified germinal epithelium showing different stages of spermatogenesis. Among the tubules, narrow interstitial spaces were identified and inhabited by clusters of interstitial cells of Leydig. The lining epithelium showed two types of cells; the spermatogenic cells and the supporting Sertoli cells. Spermatogenic cells involved spermatogonia, primary spermatocytes, spermatids and spermatozoa. Supporting Sertoli cells were present among spermatogenic cells resting on the basement membrane. They appeared as pyramidal cells with pale triangular or oval nuclei (Figure 8B). Sections of testes in the PTU treated group showed loss of normal round shape of the tubules; they appeared with irregular contour. Some seminiferous tubules were separated with wide interstitial space through which remnants of interstitial cells of Leydig were recognized (Figure 8C). Germinal epithelial layers showed gap spaces present among cells. The surrounding capsule appeared thickened with subcapsular hyaline acidophilic material deposition (Figure 8D). Sections of testes in PTU and LC treated group showed that most seminiferous tubules had round shape with regular contour. The separations between tubules became narrower than the PTU treated group (Figure 8E). Germinal epithelium in some tubules showed different stages of spermatogenesis. Spermatogenic cells included spermatogonia, primary spermatocytes, spermatids and spermatozoa. Whorls of sperms were present inside the lumina of these tubules. Other tubules showed gaps noticed among spermatogenic cells (Figure 8F).
Figure 8.Photomicrographs of testis sections of the different groups: A) Control group showing round seminiferous tubules (T) with regular contour. Tubules appear packed together with narrow interstitial space. B) Higher magnification of the previous section showing the different spermatogenic cells of the germinal epithelium including: spermatogonia (sg), primary spermatocytes (SP) and sperms (spr). Cluster of interstitial cells of Leydig (It) present in the narrow interstitial space. C) PTU-treated group showing loss of normal round seminiferous tubules (T) that appear with irregular contour. Widening of the interstitial space (Is) through which remnants of interstitial cells of Leydig (It) can be recognized. D) Section in the PTU treated group showing thick capsule (Ca) with subcapsular acidophilic hyaline material deposition (E). Germinal epithelium showed gap spaces (s) present among cells. Primary spermatocyte (Sp). E) PTU and LC treated group showing restoration of many seminiferous tubules (T) to their normal shape with regular contour. Most tubules restored its tight junction however there is still widening in the interstitial space (Is), remnants of Leydig cells (It) and gap spaces (s) between spermatogenic cells of some tubules. F) Section in the PTU and LC treated group showing that germinal epithelium begins to restore its normal cellular composition. Seminiferous tubules (T) contained whorls of sperms. However, gap spaces (S) between epithelial cells are still present. A C & E: H&E X 100; B D &F: H&E X400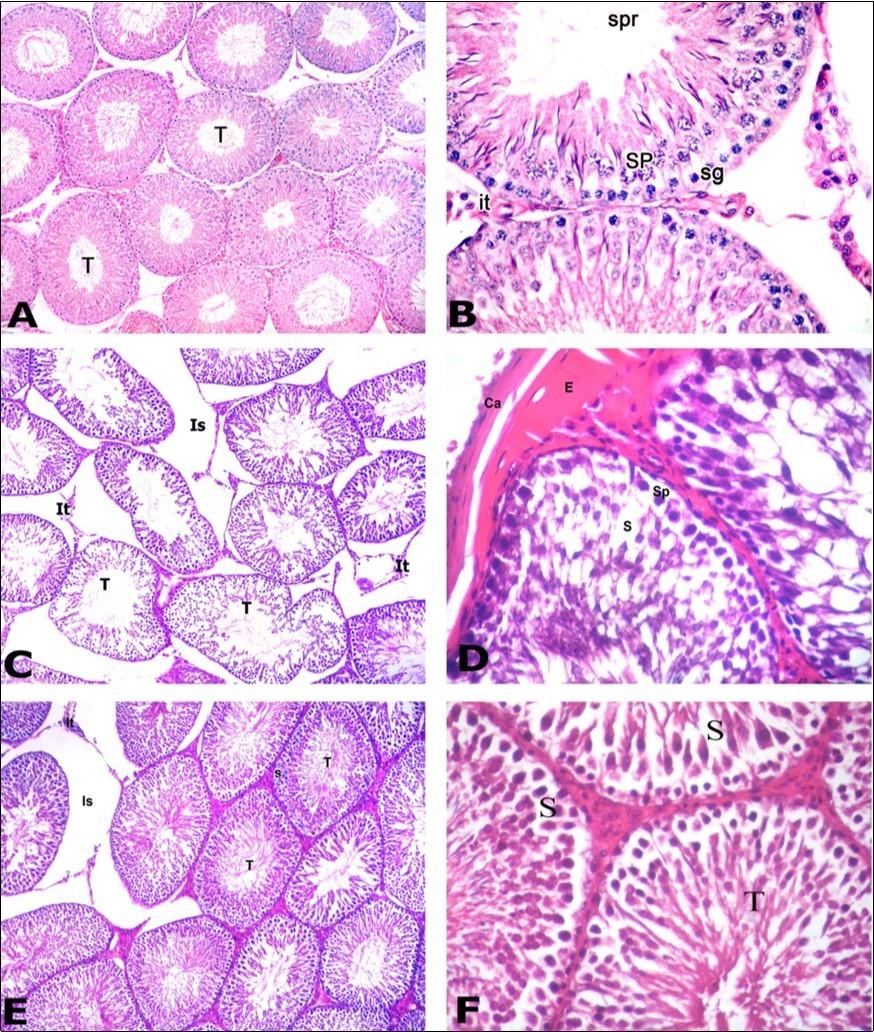
Mallory’s Trichrome Stain
Sections stained with Mallory’s trichrome stain showed normal collagen fibers distribution in the control group. The collagen fibers appeared blue in color and were located in the testicular capsule (Figure 9A). In PTU treated group, sections showed marked increase in collagen fibers distribution in the testicular capsule (Figure 9B). on the other hand, sections in PTU and LC treated group showed decrease in collagen fibres distribution in the testicular capsule in comparison to the PTU treated group (Figure 9C).
Figure 9.Photomicrographs of testis sections of different groups: A) Control group showing the normal distribution of the collagen fibers located in the capsule (arrows). B) PTU-treated group showing marked increase in collagen fibers distribution in the capsule (arrows). C) PTU and LC group showing decrease in collagen fibers distribution (arrows) in the testicular capsule in comparison to the PTU treated group. Mallory’s trichrome x 100
Immunohistochemical Staining with PCNA
Immunohistochemical staining of testicular sections in the control group showed strong positive immunostaining in most of the basal germ cells (spermatogonia) while other germ cells showed negative staining (Figure 10A). In PTU treated group, sections showed a weak immunostaining with PCNA, compared with the control group in some spermatogonia and negative immunostaining in other cells (Figure 10B). However in PTU and LC treated group showed positive immunostaining with PCNA- stronger than the PTU treated group but still weaker than the control group – in most of the spermatogonia (Figure 10C).
Figure 10.Photomicrographs of testis sections of different groups: A) Control group showing strong positive immunostaining in spermatogonia (arrows). B) PTU-treated group showing weak positive immunostaining with PCNA detected in some spermatogonia (arrows) and negative immunostaining in other spermatogonia (head arrows). C) PTU and LC-treated group showing positive immunostaining with PCNA- stronger than the PTU treated group but still weaker than the control group detected in spermatogonia (arrows). PCNA x 400
6. Morphometric Study
There was a significant difference between the studied groups regarding perimeter (on LSD comparison, the control group had the lowest value). There was also a significant difference between the studied groups regarding perimeter (on LSD comparison, PTU treated group had showed the lowest value) (Table 5, Figure 11, Figure 12).
Table 5. Morphometric study| Control group | PTU-treated group | PTU and LC-treated group | F | p | |
| Perimeter (μm)Mean ±SD(Range) | 813.8±54.18(765-856.06) | 969.3±103.79(909 – 1000.3) | 821.07±56.46(773 – 890) | 19.18 | <0.001 |
| Height germinal epithelium (μm)Mean ±SD(Range) | 91.49±2.93(30 –39.9) | 54.14 ± 1.34(53.4– 56.74 | 84.16± 4.35(79.8 – 88.2) | 106.62 | <0.001 |
Figure 11.Bar chart showing perimeter of seminiferous tubules in studied groups.
Figure 12.Bar chart showing germinal epithelium height in studied groups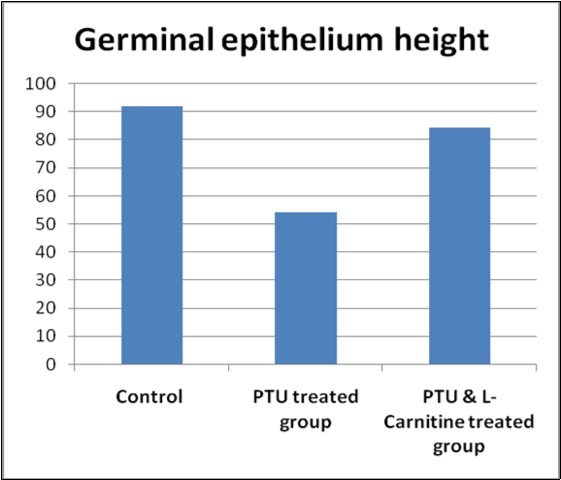
Discussion
In the present study, there was a significant increase in the rat body weight in the PTU &LC-treated group as well as control group. However, the PTU-treated group showed a decrease in the weight compared with the control group. Weight loss in the PTU-treated group indicates that the well-known weight gaining effect of hypothyroidism requires longer period to occur. These results agree with those of Ferreira et al. 13 who induced hypothyroidism in 11 mice receiving 1mg/ml/day of PTU in drinking water for 30 days; and stated that weight loss was evident with PTU as there were increased protein catabolism, decrease muscle mass, decrease absorption of carbohydrates and osteopenia. While weight gaining in the PTU and LC group agrees with Abd-Elrazek and Ahmed-Farid 12 who injected 5 rats with LC for 28 days at a dose of 350 mg/kg after receiving busulfan (anti-cancer drug that causes infertility) and stated that rat body weight increased on administration of LC against the anti-cancer therapy.
In the present study, there was no significant difference between the right and left testes weights in any investigated group. PTU-treated group showed a decrease in the testicular weight compared with the control group and this agrees with those of Choudhury et al. 18 who induced hypothyroidism in a group of rats with 0.05% PTU in drinking water for 30 days and stated that hypothyroid status caused a decrease in the testicular weight compared to the euthyroid status. LC administration caused insignificant decrease in testicular weight.
Moreover, there was no significant difference between the right and left testes volumes in any investigated group. Testes’ volumes were significantly increased in the PTU treated group compared with the control group and the PTU & LC-treated group. These results could be caused by the wide interstitial spaces and the hyaline material deposition noticed histologically in the PTU-treated group.
The levels of total T3 and T4 were significantly low in both PTU-treated and PTU & LC-treated groups compared with the control group confirming the occurrence of hypothyroid status in both groups. This indicates that LC has no effect on thyroid hormones production and any improvement that might happen is a result of local action of LC on the testicular tissue.
In the present study, the levels of GSH, CAT and SOD were significantly decreased in PTU-treated group whereas level of MDA was increased. This indicates an oxidative stress that exhausted the antioxidant defences in the testicular tissue. This oxidative stress in the PTU-treated group explained the apoptotic changes evident in the histological examination seen in the same group. These results agree with those of Choudhury et al. 18 who stated that hypothyroidism created an oxidative stress confirmed by the decrease in the level of GSH which is transformed to the oxidized form; glutathione disulfide (GSSG). The authors added that SOD and CAT are significantly decreased with significant increase in the MDA which is a sure sign for oxidative stress. Also, Sahoo et al. 9 who induced hypothyroidism in a group of rats by giving PTU 0.05% in drinking water stated that hypothyroidism lasting 30 days caused a significant drop in the GSH level with equivalent increase in the GSSG level and a significant decrease in the SOD and CAT levels.
The LC proved itself as an antioxidant enhancer against the oxidative stress caused by hypothyroidism. It reduced the MDA level in testicular tissue indicating improvement of the oxidative stress. In addition, it maintained the GSH, CAT and SOD levels near their levels in the control group and prevented their exhaustion by the stress of hypothyroidism. These results explained the improvement in the histological appearance of the protected group. Similar results were obtained by Abd-Elrazek and Ahmed-Farid 12 who stated that LC administration is accompanied by improvement in the oxidative stress. The authors added LC decreased the MDA levels in testicular tissue with increase in the GSH level and equivalent decrease in the GSSG.
On the other hand, PTU administration was shown to decrease the sperm counts significantly with a significant increase in the abnormal forms in the seminal fluid in comparison to the control group. Also, sperm motility was the minimum among the other groups. This is concomitant with the histological findings of this study indicating bad impact of oxidative stress on testicular functions. These results agree with those of Choudhury et al. 18 and Issa and El-Sherif 19.They stated that hypothyroid status decreased the total sperm count and motility significantly and increased the abnormal sperm forms and dead sperms. On the other hand, Hegazy et al. 20 attributed such hazards occurring in the spermatogenesis to the possibility of occurrence of high rates of apoptosis.
It was noticed that LC counteracted the action of hypothyroidism on seminal fluid as it elevated the sperm count and motility significantly with a significant decrease in the percentage of abnormal forms. These results agree with those of Abd-Elrazek and Ahmed-Farid 12 who stated that LC succeeded to improve the semen quality and quantity in rats after administration of busulfan. The authors added that LC improved both motility and count and diminished the abnormalities in the seminal fluid.
In the present study, H&E staining of the PTU-treated group revealed that most seminiferous tubules lost their normal shape; they appeared with irregular contour. Wide interstitial spaces were present separating the tubules containing some remnants of interstitial cells of Leydig. The germinal epithelium was significantly diminished with presence of gap spaces between its cells. The capsule was thickened with subcapsular and interstitial hyaline acidophilic material deposition. These results are similar to those reported by Tahmaz et al. 21 after injection of 20 rats with intraperitoneal PTU at a dose of 10 mg/kg for 14 days and reported that there was marked decrease in both Sertoli and Leydig cells, cessation of spermatogenesis, minimal thickening of the basement membrane and interstitial edema. Furthermore, the results agree with those of Issa and El-Sherif 19 who induced hypothyroid status in 10 rats with PTU given at a dose of 5 ml/kg for 21 days and reported that the tubules gained irregular borders, diminished germinal epithelium height and arrest of spermatogonial division with presence of halos in between cells indicating apoptotic changes. These histopathological changes were explained by the last authors as they are the results of excessive lipid peroxidation and production of ROS that indicates an oxidative stress as hypothyroidism increases carbonylation of proteins which compromises the testicular antioxidant defense. The authors added that hypothyroid status decreases the expression of testicular androgens receptors which in turn affect spermatogenesis and testosterone production negatively.
The histopathological changes were noticed to be improved in PTU & LC-treated group where most seminiferous tubules had round shape with regular contour. The separations between tubules became narrower than the PTU-treated group. Germinal epithelium in some tubules showed different stages of spermatogenesis. Spermatogenic cells included spermatogonia, primary spermatocytes, spermatids and spermatozoa. Whorls of sperms were present inside the lumina of these tubules. These hopeful results of LC administration compared with tubular damage accompanying the hypothyroid status come in consistence with those reported by Salama et al. 22 who injected 10 rats with intraperitoneal LC at a dose of 300 mg/kg for 21 days and stated that LC enhanced the spermatogenic cycles and the germinal epithelium contained spermatogonia, primary spermatocytes, spermatids and sperms. These results were explained by Abd-Elrazek and Ahmed-Farid 12 who reported that LC pronounced protective effect against infertility as LC acts as antioxidant protecting the cells against oxidative stress by removing the toxic acetyl-CoA in the cell membrane.
In the present study, Mallory’s trichrome staining of the PTU-treated group showed marked thickness in the capsule due to increased collagen fibers distribution in the capsule. These results are similar to those of Abd Elazeem et al. 23 who induced hypothyroidism in 10 rats with carbimazole at a dose of 0.05 mg/kg / day for successive 3 weeks by gastric tube and examined their parotid glands and stated that hypothyroid status caused extensive collagen fibers in between the lobules. This increase in the collagen fibers was explained by Kurus et al. 24 who stated that oxidative stress in the testicular tissue promotes excess collagen fibers production.
Mallory’s trichrome staining of the PTU&LC-treated group showed that LC administration diminished the previous effect in the PTU treated group where the collagen fibers distribution was much less in the capsule. These results could be explained by the investigators Zambrano et al. (25) who investigated the protective role of LC against hypertension and cardiac fibrosis and stated that at a dose of 400 mg /kg /day LC succeeded to reduce the elevated collagen fiber deposition (caused by hypertension and cardiac fibrosis) to its normal levels as LC inhibited the mRNA expression of collagen type one and three.
In the present study, immunohistochemical staining of the PTU-treated group with PCNA showed a weak positive staining in some spermatogonia and negative staining in other spermatogonia compared with the control group. This finding agrees with results of Zamoner et al. 10 who stated that hypothyroid status is associated with reduction in the antioxidant defense mechanisms in the testicular tissue and accumulation of the toxic ROS which disturb the cellular metabolism with subsequent apoptosis. While in PTU&LC-treated group PCNA immunohistochemical staining showed that LC was noticed to maintain and improve the cellular divisions in this group as the immunohistochemical staining became stronger than the PTU treated group. These results highlighted the antioxidant role of LC reported by Abd-Elrazek and Ahmed-Farid (12) as they described LC as a regulator for fatty acids metabolism that prevents accumulation of toxic acetyl-CoA and thus enhances the cellular functions.
Conclusions
Hypothyroidism caused changes in the histology, morphometry and principal functions of testes by creating an oxidative stress as a pathway for induction of its harmful effect. Co-administration of LC with PTU is suggested to improve the bad impact on testicular tissues via correction of the oxidative stress. More future studies with large numbers and other models of animals are recommended to ascertain beneficial role of LC in such cases before testing in human volunteers.
Acknowledgements
No funding was provided. The authors are grateful to all members of the family members of Human Anatomy and Embryology Department, for their kind co-operation.
Abbreviations
References
- 1.Hegazy A A. (2019) Human Anatomy: An Inlet of Medicine and Surgery. , International Journal of Human Anatomy 1(4), 1.
- 2.Bednarek J, Wysocki H, Sowinski J. (2004) Oxidation products and antioxidant markers in plasma of patients with Graves' disease and toxic multinodular goiter: effect of methimazole treatment. , Free Radical Research 38, 659-64.
- 3.Das K, Chainy G B. (2004) Thyroid hormone influences antioxidant defense system in adult rat brain. Neurochemical Research. 29, 1755-66.
- 4.Wajner S M, Wagner M S, Melo R C, Parreira G G, Chiarini-Garcia H et al. (2007) Type 2 iodothyronine deiodinase is highly expressed in germ cells of adult rat testis. , J Endocrinol 194, 47-54.
- 5.Venditti P, S Di Meo. (2006) Thyroid hormone-induced oxidative stress. Cellular and Molecular Life Sciences. 63(4), 414-34.
- 6.Kao S H, Chao H T, Chen H W, TIS Hwang, Liao T L et al. (2008) Increase of oxidative stress in human sperm with lower motility. , Fertil Steril 89(5), 1183-90.
- 7.Johnson F, Giulivi C. (2005) Superoxide dismutases and their impact upon human health. , Molecular Aspects of Medicine 26, 340-52.
- 8.Kücükakin B, Gögenur I, Reiter R J, Rosenberg J. (2009) Oxidative stress in relation to surgery: is there a role for the antioxidant melatonin?. , J Surg Res 152, 338-47.
- 9.Sahoo D K, Roy A, Bhanja S, Chainy G B. (2008) Hypothyroidism impairs antioxidant defense system and testicular physiology during development and maturation. , General and Comparative Endocrinology 156, 63-70.
- 10.Zamoner A, Barreto K P, Filho D W, Sell F, Woehl V M et al. (2008) Propylthiouracil-induced congenital hypothyroidism upregulates vimentin phosphorylation and depletes antioxidant defenses in immature rat testis. , Journal of Molecular Endocrinology 40, 130-4.
- 11.Abbasi S H, Heidari S, Mohammadi M R, Tabrizi M, Ghaleiha A et al. (2011) Acetyl-L-carnitine as an adjunctive therapy in the treatment of attention-deficit/hyperactivity disorder in children and adolescents: a placebo-controlled trial. , Child Psychiatry HumDev 42, 367-75.
- 12.Abd-Elrazek A M, OAH Ahmed-Farid. (2017) Protective effect of L-carnitine and L-arginine against busulfan-induced oligospermia in adult rat. , First international journal of andrology 50, 3-6.
- 13.Ferreira E, Silva A, Serakides R, Gomes A, Cassalil G. (2007) Model of induction of thyroid dysfunctions in adult female mice. , Arq. Bras. Med. Vet. Zootec 59(5), 1245-9.
- 14.Yüncü M, Bükücü N, Bayat N, Sencar L, Tarakcioglu M. (2015) The effect of vitamin E and L-carnitine against methotrexate-induced injury in rat testis. , Turk J Med Sci 45(3), 517-25.
- 16.Hegazy R, Hegazy A. (2015) Hegazy’ Simplified Method of Tissue Processing (Consuming Less Time and Chemicals). Annals of International Medical and Dental Research 1(2), 58-60.
- 17.Elias J M, Margiotta M, Gaborc D. (1989) Sensitivity and detection efficiency of the peroxidase antiperoxidase (PAP), avidin-biotin peroxidase complex (ABC) and peroxidase-labeled avidin-biotin (LAB) methods. , Am J Clin Pathol 92(1), 62-7.
- 18.Choudhury S, Chain G B, Mishro M M. (2003) Experimentally induced hypo- and hyperthyroidism influence on the antioxidant defense system in adult rat testis. , Andrologia 35(3), 131-40.
- 19.Issa N M, El-Sherif N M. (2017) Effect of Ginseng on the Testis of Subclinical Hypothyroidism Model in Adult Male Albino Rat. , Austin J Anat 4, 2-8.
- 20.Hegazy R, Hegazy A, Ammar M, Salem E. (2015) Immunohistochemical measurement and expression of Mcl-1 in infertile testes. Front Med. 9(3), 361-7.
- 21.Tahmaz L, Go¨kalp A, Kibar Y, Koc¸ak I, Yalc¸in O et al. (2000) Effect of hypothyroidism on the testes in mature rats and treatment with levothyroxine and zinc. , Andrologia 32, 85-9.
- 22.Salama A F, Kasem S M, Tousson E, Elsisy M K. (2015) Protective role of L-carnitine and vitamin E on the testis of atherosclerotic rats. , Toxicol Ind Health 31(5), 467-74.
- 23.Abd Elazeem A, Mohammed M Z, Hassan E Z. (2016) Effect of Experimentally Induced Hypothyroidism on the Parotid Gland of Adult Male Albino Rats and the possible Role of Thyroid Hormone Supplementation. , British Journal Science 14, 24-36.
Cited by (4)
This article has been cited by 4 scholarly works according to:
Citing Articles:
Cyprus Journal of Medical Sciences (2025) Crossref
Noura Essa, Bahaa Farrag, Ahmed Mohamed Kamel, mohammed Naser, Ibrahim Abd El-Hamid - Egyptian Journal of Veterinary Sciences (2024) Semantic Scholar
The Egyptian Journal of Hospital Medicine (2021) OpenAlex
N. Omar, A. A. El-Kot, E. Khalifa - (2021) Semantic Scholar
