Histological and Biochemical Study on Mitigation of Dichlorvos-Induced Hepatotoxicity by Mimosa Pudica in Mice
Abstract
Objective
Exposure of dichlorvos-contaminated foods, water and environment can lead to decrease in proper liver function. Thus, Mimosa pudica(MP)is being investigated in the present study to determine its protective effect on dichlorvos induced hepatotoxity in Mice.
Methods
Fifty adult male BALB/c mice weighing between 20-30g were randomly assigned into 5 groups of 10 animals each (Groups A, B, C, D, and E). Group A as the control Group received normal feed, group B received 0.1 ml of MP, group C was given 40 g of 2.5% Dichlorvos (DDVP) for 28 days. While, group D were given 40 g of 2.5% DDVP with 0.1ml of MP and group E animals were given DDVP for half the period of administration, normal feed and 0.1ml MP for 14 days. Histological and biochemical preparations of the liver were processed and data were expressed as mean± SEM. Significant difference was set at p<0.05.
Results
ALT activity and the total protein level of the liver show no significant increase (P < 0.005) when compared with the control. AST and ALP activities were significantly increased in animals given DDVP with subsequent MP treatment when compared with the controls. Histological studies revealed distortion of normal hepatic histoarchitecture in DDVP group B and MP groups mitigated these changes in the treated groups.
Conclusion
Dichlorvos caused tissue distortion in the mice with prominent toxic effects on the liver while MP extract showed ameliorative effects on the liver that was exposed to DDVP
Author Contributions
Academic Editor: Abdelmonem Awad Mustafa Hegazy, Professor and Former Chairman of Anatomy and Embryology Department, Faculty of Medicine, Zagazig University, Egypt.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2020 Enye Linus Anderson, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Dichlorvos is highly toxic by means of the following: inhalation, absorption through skin, and ingestion. It is very volatile, and inhalation is the most common route of exposure 1. DDVP with organophosphates is readily absorbed by dermal absorption and with the effects of cholinesterase inhibition the acute illness from DDVP is limited which causes a more rapid onset of symptoms, followed by a similarly rapid recovery according to National library of medicine 2. This occurs because DDVP is rapidly metabolized and eliminated from the body. Persons with reduced liver disorders or recent exposure to cholinesterase inhibitors will be at increased risk from exposure to DDVP but alcoholic beverages may enhance the toxic effects of dichlorvos. High environmental temperatures or exposure of DDVP to light may increase the chance of its toxicity. It also causes the skin irritation, burning sensations, or actual burns. DDVP does not occur naturally in the environment and can be used as a synthetic organic chemical for insecticide in the manufactured industries. It is effective against mushroom flies, aphids, spider mites, caterpillars, thrips, and whiteflies in greenhouse, outdoor fruit, and vegetable crops. DDVP has been shown to exhibit neurotransmitter and relaxant functions 2, 3.
MP Linn is a creeping annual or perennial herb and well known for the rapid plant movement. It contains the toxic alkaloid mimosine, which has been found to also have anti-proliferative and apoptotic effects 4. Aqueous extracts of the roots of the plant have shown significant neutralizing effects on the venom of the cobra 5 and also possess both antioxidant and antibacterial properties. MP plant has been suggested to possess low levels of toxicity. Phytochemistry has shown the compositions of MP as follows “alkaloids, flavonoid C-glycosides, sterols, terenoids, tannins, and fatty acid 6. The root of the plant has been shown to contain 10% tannin and a substance similar to adrenaline has been found within the plant leaves. The roots contain sac-like structures that release organic and organosulfur compounds including SO2, methylsulfinic acid, pyruvic acid, lactic acid, ethanesulfinic acid, propane sulfinic acid, 2-mercaptoaniline, S-propyl propane 1-thiosulfinate, and thioformaldehyde 7.
Human consumption and exposure of dichlorvos-contaminated foods, water and environment can lead to decrease in proper liver function by a decrease in enzyme activity of the liver. Thus, MP is being investigated in the present study to determine its protective, as well as its therapeutic role on liver of mice exposed to dichlorvos.
Materials and Methods
Animal Care and Management
50 Adult male BALB/c weighing between 20-30g were purchased from Afe Babalola University Ado-Ekiti (ABUAD) Farms, Ekiti, Nigeria. They were housed in well-ventilated standard cages, kept and maintained under laboratory condition of temperature, humidity and light. They were allowed to acclimatize for a period of two weeks and fed with Rat chow which was purchased from ABUAD Feed Mill, Ado-Ekiti, Ekiti State. The mice were given feed and tap water ad libitum using plastic bowls. At the end of two weeks, the mice were weighed and picked randomly and assigned to five different groups of 10 animals each; designated as; group A as the Control Group, group B as Ethanolic extract of MP Group, group C as the Toxicity Group, group D as Prophylactic Group and group E as Therapeutic Group.
Source of Plant Material
Fresh leaves of MP were collected from Afe Babalola University Farm in Afe Babalola University Ado-Ekiti, Ekiti State, Nigeria.
Extract Preparation
The fresh leaves of MP were harvested and rinsed first with distilled water and subsequently, normal saline to remove dirt and mycotoxins. The leaves were air-dried for five days under shade and then pulverized into fine powder using an electric blender (Miller iii model MS-223). 700gramme of MP was added into 2149 ml of methanol and left for two days to infiltrate. 0.4gramme of the dry MP sample was measured using a weighing balance and 20ml of distilled water was added forming the extract 8.
Dichlorvos (Sniper); (DDVP) was purchased locally for Saro Agro science Limited, Nigeria.
Food Measurement
The food intake was measured every day using the Gallenhamp electronic balance, during the period of acclimatization. The mice consumed an average of 4gramme of feed each daily.
Experimental Design
Administration of 0.1 ml volume which containing 200 mg/kg body weight of ethanol extract of MP was done orally using an insulin syringe with the aid of the cannula 9.In the experiment, the mice were grouped into 10 animals per group (5 groups) designated as A-E with an average weight of 20 - 30g. Group A as the Control was given 40gramme of normal feed and water every day for 28 days, Group B was given 40gramme of normal feed and water along with 0.1ml MP orally for 28 days, Group C was given 40gramme of 2.5% DDVP poisoned feed with water, Group D was given 40gramme of 2.5% DDVP poisoned feed and water along with 0.1ml of MP orally and Group E was given DDVP poisoned feed with water for half the period of administration (14 days) and 40gramme of normal feed and water along with 0.1ml MP orally for the remaining half (14 days) (Table 1).
Table 1. Animals’ groups and experimental design| S/N | GROUPS | AGENTS | DOSES | FOOD IN TAKE | ROUTE | DURATION |
| 1 | A | Distilled water | 0.1 ml | 40 g | Oral | 28 days |
| 2 | B | MP | 0.1 ml | 40 g | Oral | 28 days |
| 3 | C | DDVP + water | 2.5% DDVP | 40 g | Oral | 28 days |
| 4 | D | MP+ DDVP | 0.1 ml of MP+ 2.5 % DDVP | 40 g | oral | 14 days |
| 5 | E | MP + DDVP | 0.1 ml + DDVP | 40 g | Oral | 14 days |
Scarification of Animals
At the end of the experiment, the mice were sacrificed. This was done by cervical dislocation. Animals’ tissues for histological studies were perfused with normal saline and 10 % formal saline; and processed for light microscopic examination and stained with haematoxylin and eosin (H&E) 10.
Blood Collection
At the end of withdrawal period, blood was collected by cardiac puncture with the aid of non-heparized capillary tubes. About 1 ml was dispensed into clean sample bottles without anticoagulant and left to clot, to be used for biochemical studies.
Estimation of Biochemical Parameters
Blood samples collected were centrifuged and frozen at 4oC before the biochemical procedure was performed. The liver serum enzymes such as Alanine aminotransferase (ALT), Aspartate aminotransferase (AST) and Alkaline phosphatase (ALP) and Total Protein (TP)were assayed using commercially available kit (Randox, Northern Ireland).
Photomicrography
Olympus binocular microscope was used. A 5.1 megapixel MV550 research camera for microscopes was mounted in one of the oculars. This was connected to a computer running on image capture and analysis software. The immersion oil was dropped on the slide and gradually adjusted to adapt X100 oil immersion objective. The system was adjusted to obtain clarity and resolution. The image was captured and saved on the computer.
Statistical Analysis
One-way ANOVA was used to analyze data, followed by Student Newman-Keuls test for multiple comparisons. GraphPad Prism 5 (Version 5.03; Graphpad Software Inc., San Diego,CA) was the statistical package used for data analysis. Statistically significant difference was set at p<0.05.
Results
Biochemical Observations
ALT activity showed no significant effect on the liver of the administered groups when compared with the control (Figure 1). AST activity was significantly increased in animals given DDVP with subsequent MP treatment compared with the control. DDVP+MP also showed significant increase in AST level when compared with animals given MP only, and animals treated with DDVP only. No significant difference was observed in animals treated with both DDVP and Mimosa concurrently (DDVP/MP) (Figure 2).
Figure 1.shows no significant effect on the liver of the administered groups when compared with the control in ALT activity (P>0.05)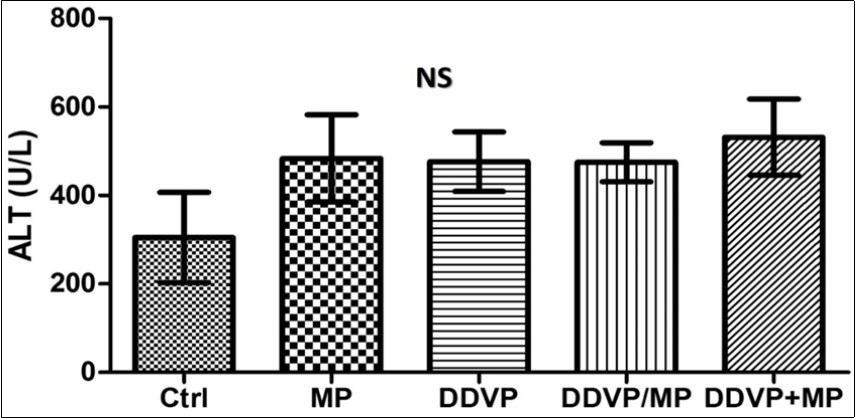
Figure 2.DDVP+MP also shows significant increase in AST level when compared with animals given Mimosa only (MP) (*P<0.05), and animals treated with DDVP only (**P<0.001). No significant difference was observed in animals treated with both DDVP and MP concurrently.
ALP activity was significantly higher in animals treated concurrently with DDVP and Mimosa (DDVP/MP) compared with control (Figure 3). No significant difference was observed in the total protein level of the liver of experimental animals (Figure 4).
Figure 3.Shows that the level of ALP was significantly higher in animals treated concurrently with DDVP and MP compared with control (*P<0.05).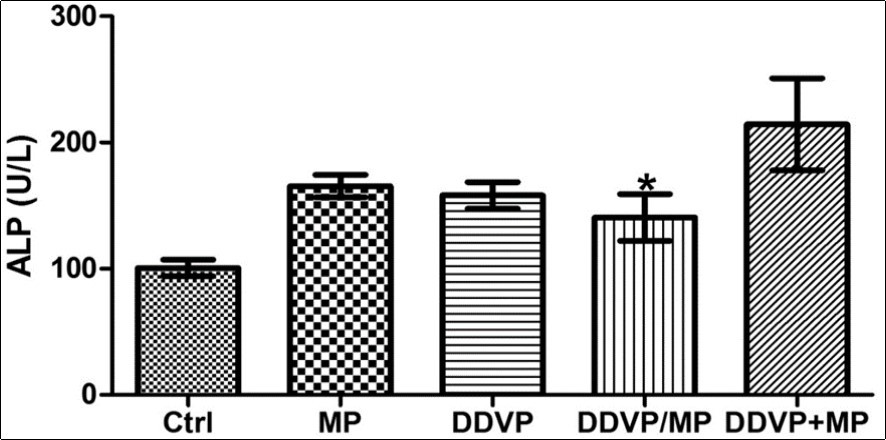
Figure 4.shows there was no significant difference observed in the total protein level of the liver of experimental animals.
Histological Findings
H&E revealed that the liver of the control group is healthy and normal as the section is free from collections and inflammatory cell (Figure 5a). MP group showed hepatic tissue composed of the liver parenchymal seperated by the sinusoids, and the central vein appear normal (Figure 5b). The group that was administered with DDVP showed marked distruption of hepatic parenchyma, increasd number of kuppfer cells, congested central vein and moderate infilteration of the inflammatory cells within the interstitium (Figure 5c). The group that was administered with DDVP first then MP after showed perivascular and interstitial infilteration of inflammatory cells. The hepatocytes and sinusoids appeared normal (Fig. 5d). The group that was administered with DDVP and MP concurrently showed marked periportal inflammatory cell infilterate, marked distruption of the hepatic parenchymal microanatomy (Figure 5e).
Figure 5.(A-E): Photomicrographs of Liver show the hepatic tissues composed of hepatocytes (H) disposed in sheet, the hepatocytes are seperated by the sinusoids (S). the central vein (CV) is well outlined in (A Control) Section is free from collections and inflamatory cells. (B) Arrow head pointed to kuppfer cells, the central vein appear normal. (C) There was distruption of hepatic parenchyma (circle), increasd number of kuppfer cells (arrow head). (D) perivascular and interstitial infilteration of inflammatory cells (arrow). The hepatocyte (H) and sinusoids appear normal. (E) periportal inflammatory cell infilterate (arrow), marked distruption of the hepatic parenchymal microanatomy (circle). H&E (x400)
Discussion
This study used male animals to avoid the possible hormonal changes occurring in females’ oestrus cycles 11. Exposure to DDVP and MP simultaneously caused a decline in the health of the liver of treated animals which is ameliorated with MP treatment. A normal liver is healthy and free from collections and inflammatory cell 12. Several studies have shown that dichlorvos affects proper liver function during exposure 2 as DDVP is a known toxic organophosphate. The group that was fed with DDVP showed marked disruption of hepatic parenchyma, increased number of Kupffer cells, congested central vein and moderate infiltration of the inflammatory cells within the interstitial; while MP group showed hepatic tissue composed of the liver parenchyma separated by the sinusoids; Kupffer cells and the central vein appeared normal. This shows the ameliorative properties of MP, which may be due to the antioxidant properties associated with MP 13. The group that was administered with DDVP and MP concurrently showed marked periportal inflammatory cell infilterate, marked disruption of the hepatic parenchymal microanatomy as MP is not able to sufficiently ameliorate the toxic effect of DDVP as they were administered together.
The alanine aminotransferase (ALT) is a blood test that checks for liver damage 14. This enzyme is found mainly in the liver. In this study, ALT activity showed no significant effect on the liver of the administered groups when compared with the control this is because while ALT is a very versatile marker for liver pathologies, it does have its limits. It’s rarely specific, and there are many cases of liver pathology without corresponding increases in serum ALT. The body uses ALT to break down food into energy. Normally, ALT levels in the blood are low, if the liver is damaged, it will release more ALT into the blood and levels will rise. The normal value for ALT in blood is between 7 and 55 units per litre.
AST is an enzyme produced by the liver. Normally, AST levels in the blood are low. When the liver is damaged, it puts more AST into the blood, and increases the AST levels 14. AST activity was significantly increased in animals given DDVP with subsequent Mimosa treatment (DDVP+MP) compared with the control. DDVP+MP also shows significant increase in AST level when compared with animals given MP, and animals treated with DDVP only. This showed that there is significant liver damage caused by continued DDVP exposure. A high AST level is a sign of liver damage No significant difference was observed in animals treated with both dichlorvos and Mimosa concurrently (DDVP/MP).
ALP activity was significantly higher in animals treated concurrently with dichlorvos and Mimosa (DDVP/MP) compared with control. An alkaline phosphatase level test (ALP) measures the amount of alkaline phosphatase enzyme in your bloodstream. Abnormal levels of ALP in your blood most often indicate a problem with liver 14.
Total protein test is used to measure the total amount albumin and globulin in your bodyincrease in TP indicates the presence of liver disease. In this study, no significant difference was observed in the total protein level of the liver of experimental animals.
Conclusion
This study showed that dichlorvos caused tissue distortion in the mice with prominent toxic effects on the liver. The mimosa extract showed ameliorative effects on the liver that was exposed to DDVP.
Authors Contributions
ELA design and supervised the research. EE analyzed, interpreted the result and drafting the article. KA and AG provided research materials and organized data. SOS analyzed and helped in drafting the article. All authors reviewed and approved the final draft of the manuscript.
Acknowledgment
The authors are grateful to management of Afe Babalola University for providing a good environment to conduct this research. Authors are very grateful to Mr Fafure AA, Mr Adedeji A, Mrs kunlere O, Miss Nebo K for technical help, and support for this work and the MP plant was identified by Mr Omotayo Felix from the botany department of Ekiti state university.
References
- 1.Gallo M A, Lawryk N J. (1991) Organic phosphorus pesticides.In Handbook of Pesticide Toxicology. , New York, NY 5-3.
- 3.Kumar V, Basniwal P K, Vijayaraghavan R. (2018) Effect of combined exposure of dichlorvos and monocrotophos on neurotransmitters and acetylcholinesterase in rat. , Int J Pharm Sci& Res 9(11), 4956-4962.
- 4.Restivo A, Brard L, Granai C O, Swamy N. (2005) Antiproliferative effect of mimosine in ovarian cancer. , Journal of clinical oncology 23(16), 3200.
- 5.Elmerich C, Newton W E. (2007) Associative and endophytic nitrogen- fixing bacteria and cyanobacterial associations.Springer. 1-20.
- 6.Genest S K, Shah A, Rahman M M, Saif E, Naser G M et al. (2008) Comparative bioactivity of two Mimosa species. Lat Am Caribb Bull Med Aromat Plants. 7, 38-43.
- 7.Musah R A, Ashton DM Lesiak, Max J C, Robert B, David F Edwards et al. (2016) Mechanosensitivity Below Ground” Touch-Sensitive Smell-Producing Roots in the "Shy Plant," Mimosa pudica L. Plant physiol. 170(2), 1075-1089.
- 8.WFW Fadzlie, Mohd E K, Zulfazli M S, Arbakariya B A. (2018) The Potential ofMimosapudicaas a Biopreservative for Food Products: a Bio processing Perspective. Nutri Food Sci Int. 5(3), 1-4.
- 9.Hegazy R, Hegazy A. (2015) . Simplified Method of Tissue Processing (Consuming Less Time and Chemicals). Annals of International Medical and Dental Research 1(2), 57-61.
- 10.Hegazy A A, Ahmed M M, Shehata M A, Abdelfattah M M. (2018) Changes in Rat’s Liver Structure Induced by Zinc Oxide Nanoparticles and the Possible Protective Role of Vitamin E. , International Journal of Human Anatomy; 1(3), 1-16.
- 11.Edem E, Enye L, Towobola A, Akingdade A, Kunlere O. (2015) The Impact of Mimosa pudica on the Histoarchitecture of Hypothalamic-Pituitary-Testicular Axis in Cadmium- treated Rats. World Journal of Pharmacy and Pharmaceutical Sciences 4(10), 169-179.
- 12.Thouas G A, Dominguez F, Green M P, Vilella F, Simon C et al. (2015) Soluble ligands and their receptors in human embryo development and implantation. Edocrine reviews. 36(1), 92-130.
Cited by (2)
- 1.Anderson Enye Linus, Stephen Saka Olusola, Udi Onoriode Andrew, Oladunni Ebeye Abimbola, Sunday Igbigbi Patrick, 2023, Investigating the effect of Mimosa Pudica on dichlorvos induced hippocampal neurodegeneration in mice, Phytomedicine Plus, 3(1), 100393, 10.1016/j.phyplu.2022.100393
- 2.Enye L. A., Ebeye A. O., Udi O. A., Ishola A. O., Igbigbi P. S., 2021, <i>Mimosa pudica</i> Ameliorated Dichlorvos Induced Neuro-oxidation, Toxicology International, (), 203, 10.18311/ti/2021/v28i3/26728
