Thymic Hypertrophy and Sudden Unexpected Death In Adults –A Retrospective Study Of 56 Autopsy Cases
Abstract
Status thymico-lymphaticus had ever been explained as a cause of sudden death usually in children, but few cases were reported in adults. We sought to determine the relationship between thymic hypertrophy and sudden unexpected death in adult (SUDA), and associated macroscopic and microscopic findings. Adult post mortems from 1984 to 2014 were reviewed and 23 thymic hypertrophy patients without SUDA, 33 thymic hypertrophy patients with SUDA and 172 SUDAs without thymic hypertrophy entered. The data of thymus, lymph nodes, spleen, heart, aorta, and adrenal glands were collected for macroscopic and histological analysis. Ten antibodies were used and applied to 3 children and 46 adult thymus specimens. We found, as an independent factor, thymic hypertrophy increased significantly the risk of SUDA (6.9 folds) in both male and female. What’s more, SUDAs associated with thymic hypertrophy were quite younger (22.5 years) than those without it. A majority of patients with hypertrophic thymus had a variable number of accompanied anomalies described as the typical characteristics of status thymico-lymphaticus, but no macroscopic and microscopic findings related to SUDA in patients with thymic hypertrophy. Cytokeratins (CKs) showed distinctly different immunohistochemical expression patterns in individuals who had different death causes and disease background. Instead of a disease entity “status thymico-lymphaticus” is a systematic abnormality with thymic hypertrophy as a feature involving mainly immune and/or cardiovascular system, probably caused by gene mutations.
Author Contributions
Academic Editor: Hesham N. Mustafa, Department of Biological Sciences, Allergan, Inc., 2525 Dupont Drive, Irvine, CA 92612-1599.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2017 Rong Zhu, et al
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Status thymico-lymphaticus, also termed as “status lymphaticus”or simply “lymphatism”, was first used in the publications in 1911, whose most important feature and essential diagnostic sign is enlargement of the thymus. This condition involves a combination of constitutional anomalies: hypertrophy of the thymus, general hyperplasia of the lymphatic system (such as the spleen and lymph nodes), hypoplasia of the cardiovascular system with aortic narrowing, and hypoadrenal. The condition is sometimes terminated by sudden death usually in children 1, 2, but few cases were reported in adults. Whether hypertrophic thymus increases the risk of the sudden unexpected death in adult (SUDA), the frequency of presence of the other anomalies, and the relationship between clinical, macroscopic and microscopic findings, there was no systemic study especially in Chinese. On the other hand, although status thymico-lymphaticus had ever been explained as a cause of SUD in patients without other determined positive anatomic findings, by now, some were questioning whether it really existed as a disease or a pathological entity.
The thymus is a lobulated organ covered by a capsule. It enlarges during childhood, and atrophies at puberty. The normal weight of the thymus varies with the age 3. As one ages the thymus slowly shrinks, eventually degenerating into tiny islands of fatty tissue. The thymus of older people is scarcely distinguishable from surrounding fatty tissue. The criteria used to identify hypertrophy of thymus are complicated and challengeable, but we can’t dodge. Thymic hypertrophy is defined as thymic enlargement beyond the upper limits of normal for the age but accompanied by a microscopically normal gland. As usual, the weight of the thymus is usually used for determination of hypertrophy. In few previous studies the great variations had been found in the weight of the normal adult thymuses, maximum weights are difficult to define. The established or recommended size and weight criteria for normal thymus are still controversial. Combined of the data in Chinese people and the Surgical Pathology Criteria from Stanford University School of Medicine (http://surgpathcriteria.stanford.edu/), we used >35g for 18-25 years, >25g for 26-39 years and >15g for 40-60 years as the criteria of thymic hypertrophy.
In this study, the deaths which had no clear cause found at post mortem were classed as “unexplained”. Of all the cases, 7 were diagnosed as “sudden manhood death syndrome (SMDS)”. SMDS is defined as a disorder found in Southeast Asia, particularly Thailand, Philippines and Japan, which causes sudden cardiac death during sleep with known or unknown cardiac disease 4. Such a rapid death is often attributed to a cardiac arrhythmia and has no apparent structural cardiac disease in some cases. For seven cases diagnosed as SMDS in this study, we classified three of them which had no positive pathological findings of cardiac disease into “unexplained” and the other four cases which had definite positive cardiac lesions into “Cardiovascular diseases”.
In recent years, previously undetected cardiac or other lesions, readily demonstrated at autopsy, have changed our understanding of SUD and status thymico-lymphaticus. By review of post mortems of 30 years, we found, thymic hypertrophy increased significantly the risk of SUDA (6.9 folds) in both male and female. What’s more, SUDAs associated with thymic hypertrophy were quite younger (22.5 years) than those without it. Instead of a disease entity “status thymico-lymphaticus” is a systematic abnormality with thymic hypertrophy as a feature involving mainly immune and/or cardiovascular system.
Materials and Methods
Cases and Definitions
All adult post mortems performed at Department of Pathology, School of Basic Medical Science, Fudan University between August 1984 and July 2014 were assessed from postmortem reports. All the autopsies were carried out within 48h of death according to a local protocol. Any cases that were incomplete, outstanding or limited to the brain were excluded. Totally, there were 2066 cases (Male: Female 1101:965) with the age more than 18 years enrolled, whose toxicology and detailed examination of the heart for structural abnormalities was negative. Sudden death (SD) was defined sudden unexpected death of adolescents and adults, many during sleep. In witnessed cases, it was defined as an acute change in cardiovascular status with time to death being <1 hour; in unwitnessed cases, SD was defined as a person last seen alive and functioning normally <24 hours before being found dead 2.
As the typical characteristics described of status thymico-lymphaticus, the data of thymus, lymph nodes, spleen, heart, aorta, and adrenal glands were collected and reviewed for macroscopic and histological analysis. The average weights of various organs varied with race and population types. Briefly, weights of most organs in Chinese adults are relatively lighter than those in Westerners. In this study, according to the studies in Chinese adults 5, 6 and our own data, the weight of the heart <264g in male or <239g in female was defined as hypoplasia; the weight of the spleen > 226g in male or >214g in female was defined as hyperplasia; the combined weight of the adrenal glands <8.9g in male or <8.7g in female was defined as hypoplasia; the width of the aorta at the beginning <5.65cm was defined as narrowing.
Immunohistochemistry and Pathological Evaluation
Formalin-fixed, paraffin-embedded thymus samples obtained at autopsy were collected for two-step immunohistochemical staining as previously described by our group 7. Ten primary ready-to-use antibodies were used: anti-CK7, -CK8, -CK10, -CK18, -CK19, -CK20, -CD3, -CD20, -TdT, and -Bcl-2, which were all purchased from Shanghai JIEHAO Biological Technology Co., LTD (Shanghai, China). The stained sections were evaluated by KS 400 Imaging System, Release 3.0, ZEISS (Oberkochen, Germany). The diagnoses were made independently by two experienced pathologists. Deaths which could not be accurately classified were reviewed by a consultant pathologist.
Results
Anatomic Distribution of Thymic Hypertrophy in Chinese Patients With and Without SUDA
There were a total of 56 cases (Male: Female 37:19) qualified for thymic hypertrophy, of which 33 were SUDA. The frequency of SUDA in patients with thymic hypertrophy was significantly higher than those without thymic hypertrophy (58.93% vs. 8.56%, x2=154.661, P<0.001). There was a more male predominance in patients with SUDA than without SUDA (Male: Female 138:67 vs. 963:898, x2=17.986, P<0.001). Likewise, thymus hypertrophy also presented a certain male preference (Male: Female 37:19 vs. 1065:945, x2=3.749, P=0.058). In patients qualified for thymic hypertrophy, there was no sex difference between those with SUDA and without SUDA (Male: Female 21:12 vs. 16:7, x2=0.213, P=0.777), and thymic hypertrophy significantly increased SUDA risk in both male and female, which was tested further by chi square test-stratified analysis (x2=2.719, P=0.099).
Macroscopic Findings
In the present study, all thymuses from the 56 cases we collected had lobular appearance and with complete capsule. The thymic tumors including thymoma and lymphoma were excluded. We divided the cases into three groups: (1) Thymic hypertrophy patients without SUDA, (2) Thymic hypertrophy patients with SUDA and (3) SUDAs without thymic hypertrophy (Table 1). No statistically significant difference was found between thymic hypertrophy patients with and without SUDA on age, sex, height, weight of the thymus, hyperplasia of the lymph nodes, weight of the spleen, weight of the heart, width of the aorta, and weight of the adrenal glands. Of all 56 patients with thymic hypertrophy, meanwhile, 16 had one, 13 had two, 8 had three and 6 had four other “status lymphaticus” associated anomalies (hyperplasia of the lymph nodes and spleen, hypoplasia of the heart and adrenal glands, and aorta narrowing), and 13 patients had no other anomalies except for thymic hypertrophy. In the thymic hypertrophy patients with both hypoplasia of the heart and aorta narrowing, there were relatively more SUDAs compared to the cases without both of them or only with one of them (72.73% vs. 48.89%, x2=2.020, P=0.155), but which didn’t reach a statistic significance. Then we compared SUDAs with and without thymic hypertrophy, and found patients with thymic hypertrophy had an age of death 22.5 years less than those without thymic hypertrophy, and meanwhile had more frequency of lymph node hyperplasia, spleen hyperplasia, heart hypoplasia, aorta narrowing and adrenal gland hypoplasia. Accordingly, the patients presenting SUDA with thymic hypertrophy had more spleen weight, less heart weight and aorta width than those without it.
Table 1. Macroscopic findings of thymic hypertrophy patients with or without SUDA and SUDAs without thymic hypertrophy.| Thymic hypertrophy without SUDA (n=23) | Thymic hypertrophywith SUDA(n=33) | SUDAs withoutthymic hypertrophy(n=172) | ||
| Age years (mean ± SD) | 29.65 ± 10.16 | 32.48±9.36 | 54.97 ±16.92 | |
| t=-1.076, P=0.287 | t=7.210, P<0.001** | |||
| Sex(male: female) | 16:7 | 21:12 | 117:55 | |
| x2=0.213, P:1.777 | x2=0.242, P=0.623 | |||
| Heightcm (mean ± SD) | 167.22 ±8.93 | 166.27 ±9.88 | 166.69 ± 8.68 | |
| t=0.366, P=0.716 | t=0.224, p=0.823 | |||
| Thymus weight g (mean ± SD) | 48.00 ± 33.73 | 52.35 ± 75.64 | not available | |
| t=-0.258, P=0.798 | not available | |||
| Hyperplasia of the lymph nodes n(%)# | 13 (56.52%) | 11 (33.33%) | 4 (2.33%) | |
| x2=2.976. P=0.085 | x2=39.255, P<0.001** | |||
| Spleen weight g (mean ± SD) | 240.35 ±115.56 | 256.56± 130.03 | 180.55 ±106.93 | |
| t=-0.480, P=0.633 | t=-3.257, P3=0.001 | |||
| Hyperplasia of thespleen n (%) | 11 (47.83%) | 15 (45.45%) | 40 (23.26%) | |
| x2=0.031, P.861 | x2=5.950, P31.008** | |||
| Heart weightg (mean ± SD) | 318.00 ±85. 00 | 308.47± 78.02 | 398.45 ±109.21 | |
| t=0.433, P=0.666 | t=4.311, P<0.001** | |||
| Hypoplasia of theheart n (%) | 7 (30.43%) | 11 (33.33%) | 14 (8.14%) | |
| x2=0.052, P=0.819 | x2=16.412, P<0.001 ** | |||
| Aorta width△cm (mean ± SD) | 6.14±0.77 | 5.93 ±0.90 | 6.97 ±1.21 | |
| t=0.876, P=0.385 | t=4.460, P<0.001** | |||
| Aorta narrowingn (%) | 7 (34.78%) | 10 (30.30%) | 18 (10.47%) | |
| x2=0.125, P=0.724 | x2=9.240. P=0.002** | |||
| Combined adrenalweight g(mean± SD) | 19.23±10.92 | 15.33±6.70 | 22.79 ±20.81 | |
| t =1.566, P=0.124 | t=1.918, P=0.058 | |||
| Hypoplasia of theadrenal glandsn (%) | 4 (17.39%) | 7 (21.21%) | 2 (1.16%) | |
| x2=0.125, P=0.723 | x2=25.639. P<0.001** | |||
Histological Findings
Microscopically, the thymuses in this study showed three conditions: non-involuted thymus, incomplete-involuted thymus and thymic hyperplasia. The uninvoluted thymus showed outer lymphocyte rich cortex and inner lymphocyte-poor medulla with Hassall corpuscles. Actually most thymuses showed various degrees of involution, including loss of septa, smaller lobules, a reduced number of lymphocytes, increased adipose tissue and epithelial nests, and a decreased number of the Hassall corpuscle. There was variable amount of fat replacement (0-80% area percent) in the thymuses. Area fraction of the fat correlated positively with the age (r=0.729, p<0.001), but didn’t correlate with SUDA, sex, weight of the thymus, and other combined anomalies or changes. In a few cases, lymph follicles (5/33 vs. 1/23, x2=1.654, P=0.198) and calcification of the Hassall corpuscle (11/33 vs. 7/23, x2=0.052, P=0.819) were seen, but there were no significance between the patients with and without SUDA.
The microscopic changes of the heart were nonspecific, and included myocardial fatty infiltration (5/23 vs. 9/33, x2=0.224, P=0.638; 9/33 vs. 55/172, x2=0.285, P=0.593), interstitial fibrosis (1/23 vs. 1/33, x2=0.068, P=0.794; 1/33 vs. 20/172, x2=2.226, P=0.136) and lipofusin in cardial myocytes (1/23 vs. 2/33, x2=0.078, P=0.779; 2/33 vs. 31/172, x2=3.593, P=0.058), which were not different between the groups. Vacuolization of the adrenal cortex presented more often in thymic hypertrophy patients with than without SUDA (6/33 vs. 0/23, x2=4.684, P=0.030), but no difference between SUDAs with and without thymic hypertrophy (6/33 vs. 27/172, x2=0.127, P=0.722). Four SUDAs without thymic hypertrophy and one with thymic hypertrophy show nodular hyperplasia of the adrenal cortex. Except for congestion in most cases and thickened vascular walls in several cases, the spleen was unremarkable in this study.
Causes of Death
The causes of death in three groups were summarized in Table 2. In the SUDAs without thymic hypertrophy group, cardiovascular diseases accounted for 71.51% of deaths versus 21.21% of deaths in the group with thymic hypertrophy (x2=15.404, P<0.001). Most thymic hypertrophy patients without SUDA died of infectious diseases (73.91%) and immune-related diseases (13.04%) including some autoimmune diseases, but no one died of cardiovascular diseases. Thymic hypertrophy patients with SUDA had equally distributed causes of death: unexplained, cardiovascular, infectious, immune-related and other diseases proximately averaged the causes of death. Considering most “unexplained” cases were probably caused by cardiac arrhythmia, which may not have apparent structural heart disease 8, cardiovascular diseases accounted for a little more SUDAs than other causes in patients with thymic hypertrophy.
Except two patients with thymic hypertrophy died directly of autoimmune diseases (Crohn’s disease and myasthenia gravis) and their complications, three patients with thymic hypertrophy and SUDA (two died of infectious diseases and one had unexplained cause of death) also presented autoimmune diseases: thrombocytopenic purpura and chronic lymphocyte thyroiditis (Hashimoto's disease), which weren’t shown on Table 2. Accompanied adrenal tumors were seen in the patients with thymic hypertrophy: one patient died of pneumonia and was accompanied by adrenocortical adenoma; meanwhile two patients with cardiac sudden death were accompanied by adrenal pheochromocytoma. In another patient with thymic hypertrophy and SUDA, who died of pneumonia and pulmonary hemorrhage, we found the right hypoplastic kidney at post mortem. More deaths were caused by acute hemorrhagic pancreatitis in patients with thymic hypertrophy (4/56, 7.14%) than without it (2/172, 1.16%).
Table 2. Causes of death in thymic hypertrophy patients with or without SUDA and SUDAs without thymic hypertrophy.| Causes of death | Thymic hypertrophy without SUDA(n=23) | Thymic hypertrophy with SUDA (n=33) | SUDAs without thymic hypertrophy(n=172) |
| Unexplained | 0 (0.00%) | 7 (21.21%) | 6 (3.49%) |
| Cardiovascular diseases | 0 (0.00%) | 7 (21.21%) | 123 (71.51%) |
| Ischemic heart disease# | 0 | 3 | 46 |
| Aneurysma with rupture | 0 | 0 | 28 |
| Cardiomyopathy | 0 | 3 | 0 |
| Sinoatria lesion | 0 | 1 | 2 |
| Pulmonary embolism | 0 | 0 | 24 |
| Hypertensive heart disease | 0 | 0 | 6 |
| Hypertensive brain disease | 0 | 0 | 17 |
| Infectious diseases | 17 (73.91%) | 6 (18.18%) | 19 (11.05%) |
| Myocarditis | 6 | 1 | 8 |
| Pericarditis | 1 | 1 | 3 |
| Pneumonia | 2 | 0 | 2 |
| Sphagitis | 0 | 1 | 5 |
| Gastroenteritis | 1 | 2 | 0 |
| Tuberculosis | 2 | 0 | 0 |
| Bacillary dysentery | 3 | 0 | 0 |
| Systematic infection | 2 | 1 | 1 |
| Immune-related diseases△ | 3 (13.04%) | 6 (18.18%) | 13 (7.56%) |
| Allergic shock | 0 | 3 | 5 |
| Acute respiratory distress syndrome | 0 | 2 | 6 |
| Asthma | 1 | 1 | 0 |
| Crohn’s disease with perforation | 1 | 0 | 0 |
| Grave’s disease | 0 | 0 | 2 |
| Myasthenia gravis | 1 | 0 | 0 |
| Others | 3 (13.04%) | 7 (21.21%) | 11 (6.40%) |
| Acute hemorrhagic pancreatitis | 1 | 3 | 2 |
| Ectopic pregnancy | 0 | 2 | 2 |
| Hemorrhage after gastrectomy | 0 | 1 | 0 |
| Peptic ulcer with hemorrhage | 0 | 0 | 2 |
| Epilepsy during operation | 0 | 1 | 0 |
| Congenital megacolon | 1 | 0 | 0 |
| Methanol toxicity | 1 | 0 | 0 |
| Bronchial foreign bodies | 0 | 0 | 5 |
Immunohistochemical Findings
Three thymus specimens obtained at autopsy, from children aged 12-18 months, were also used for immunohistochemistry. Immunoreactivity was reported in the four regions: subcapsular cell, cortex, medulla and Hassall corpuscle. All the findings described here are summarized in Table 3. Overall, CKs expression didn’t show prominent difference among the three groups. Except CK19, all other CKs (CK7, CK8, CK10, CK18, CK20) were negative for subcapsular and cortex cells. Medulla epithelial cells and Hassall corpuscles expressed almost all the CKs. A few scattered and weakly stained CK10-positive medullary cells presented in half of the adult thymuses while they were hardly seen in the other adult and child thymuses. All epithelial cells were positive for CK19. The number of CK19-positive subcapsular cells was slightly fewer in child thymuses than that seen in adult thymuses. However, CKs showed apparent variance of expression patterns in individuals who had different death causes and diagnosed for different diseases (Figure 1), which indicated that the thymic microenvironment was changeable under different conditions.
Table 3. Summary of immunohistochemical expressions in thymuses from 3 children, 19 thymic hypertrophy patients without SUDA and 27 thymic hypertrophy patients with SUDAa| C | CK7 | CK8 | CK10 | CK18 | CK19 | CK20 | Bcl-2 | TdT | CD3 | CD20 | |
| Subcapsular cell | Children | - | - | - | - | + | - | - | - | - | - |
| Thymic hypertrophy without SUDA | - | - | - | - | + | - | - | - | - | - | |
| Thymic hypertrophy with SUDA | - | - | - | - | ++ | - | - | - | - | - | |
| Cortex | Children | - | - | - | - | + | - | ± | + | ++ | - |
| Thymic hypertrophy without SUDA | - | - | - | - | + | - | ± | - | + | ± | |
| Thymic hypertrophy with SUDA | - | - | - | - | + | - | ± | - | + | ± | |
| Medulla | Children | ± | ± | - | ± | ++ | ± | + | - | ++ | + |
| Thymic hypertrophy without SUDA | ±~+ | ± | -~± | + | ++ | ± | + | - | ++ | + | |
| Thymic hypertrophy with SUDA | ±~+ | + | -~± | ± | ++ | ± | + | - | ++ | + | |
| Hassall corpuscle | Children | + | + | ++ | + | ++ | + | - | - | - | - |
| Thymic hypertrophy without SUDA | + | + | ++ | + | ++ | + | - | - | - | - | |
| Thymic hypertrophy with SUDA | + | ±~+ | ++ | + | ++ | + | - | - | - | - | |
Figure 1.Different patterns of CK19 expression in thymuses. Nuclei were stained in blue by hematoxylin and positive reaction appeared brown. A, Thymus from a 16-month child who died of interstitial pneumonia. The whole epithelial network expressed CK19 forming a meshwork structure in the cortex and the medulla (original magnification, ×200). B, The number of CK19-positive subcapsular cells was more in the thymus from a female of 29 years old with SUDA, who diagnosed as thrombocytopenic purpura and died of infectious shock, than that seen in the child thymus (original magnification, ×200). C, Thymus from a 27-year old man with cardiac sudden death and accompanied by adrenal pheochromocytoma. Reactivity with CK19 was little or absent in the medulla (△) (original magnification, ×200). D, A female of 20 years old who died of Crohn’s disease with perforation. B cells presented nodular hyperplasia and formation of lymphoid follicles (*). Their presence was accompanied by a disorderly arrangement and hypertrophy of medullary epithelial cells. The pushed epithelial cells were packed and showed strong CK19 expression (original magnification, ×100).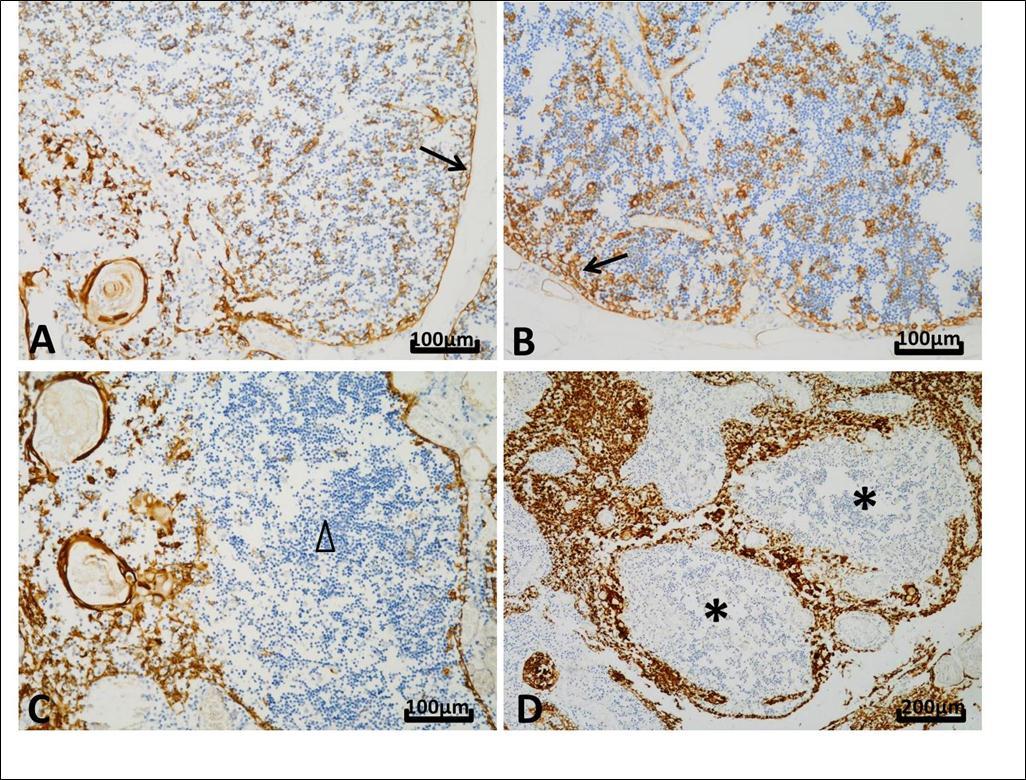
As a regulator of apoptosis, Bcl-2 was scatteredly stained on both lymphocytes and epithelial cells in cortex and medulla. TdT expression was present only in the cortex in the child thymus but absent in all adult thymuses. There were more CD3-positive T cells in cortex in child thymuses than adult thymuses. Instead of a decreased number of cortex T cells related to physiologic (age) involution, CD20-positive B cells presented in cortex. The ratios of T to B cells in medulla didn’t show obviously difference in all groups.
Discussions
We found, as an independent factor, thymic hypertrophy increased significantly the risk of SUDA (6.9 folds) in both male and female. What’s more, SUDAs associated with thymic hypertrophy were quite younger (22.5 years) than those without it. As same as SUDA, thymus hypertrophy had male predominance. It was thought that the thymus atrophy is due to the increased circulating level of sex hormones, and chemical or physical castration of an adult results in the thymus increasing in size and activity 9. So we are not surprised to see that histologic characteristics of the thymus are not identical in the two sexes 10, and it is likely to attribute the sex difference in the present study to the levels of sex hormones. In the previous studies the great variations had been found in the weight of the normal adult thymus, which was explained by intermingled parenchyma and fat in the involuted thymuses, and it was difficult to decide what to weigh 10, 11. In this study, the corrected weight of thymus was also calculated in 10 low-power fields by the weight of thymus × (1- the area of mediastinal fat %). We found the thymic weight correlated positively to its corrected weight (r=0.769, p<0.001), and both the thymic weight (r=-0.419, p=0.009) and the corrected weight (r=-0.701, p<0.001) correlated negatively to the age. In the same age group, the weight of thymus reflects the amount of thymic parenchyma and is convenient to be used as an indicator for thymic hypertrophy.
Although a majority of patients with hypertrophic thymus had a variable number of accompanied anomalies described as the typical characteristics of status thymico-lymphaticus, for example hyperplasia of the lymph nodes and spleen, hypoplasia of the heart and adrenal glands, and aorta narrowing, all of them were not associated with SUDA, because the significant differences existed only between SUDAs with and without thymic hypertrophy groups, but not between thymic hypertrophy with and without SUDA groups. Other congenital anomalies were also found on the patient with thymic hypertrophy, for example hypoplasia of the kidney and congenital megacolon, though they were rare if compared with lymphaticus-associated anomalies. No disease conditions and deaths were identified for these accompanied anomalies. Our date showed that cardiovascular diseases accounted for a little more SUDAs than other causes in patients with thymic hypertrophy. The association between cardiovascular diseases and thymic weights were also reported by Kendall. He found the deaths where cardiovascular conditions were the cause of death or a contributory factor in the death related with higher thymic wet and lipid weights 3. Mutations of the cardiac ryanodine receptor type 2 (RyR2) gene are known to cause fatal ventricular arrhythmia, syncope and sudden death. When Nishio investigated RyR2 mutations in 18 SUD autopsy cases, he found all the 2 cases with the heterozygous missense RyR2 mutation displayed characteristics of status thymico-lymphaticus. It was possible that the mutation is involved in the hypertrophy of the thymus 11. The multiple anomalies in patients with thymic hypertrophy may be caused by gene mutations. However, which mutations, single-gene or combined mutation, and their relationship with various clinical features and SUDAs still remain unclear.
The unexpected finding was the correlation between adrenal glands and thymus. Vacuolization of the adrenal cortex presented more often in thymic hypertrophy patients with SUDA than those without SUDA, however no difference between SUDAs with and without thymic hypertrophy. So we prefer it as a change related with SUDA rather than a combined anomaly of the thymic hypertrophy. It was documented that the vacuolization seems to indicate glandular hyperactivity for hormone production, and the hypothalamic-pituitary-adrenal axis can respond to stress with an increase in the production of cortisol 12. Reduced adrenal weight with thinning of the cortex and thymomegaly with hyperplasia of the thymus cortex was observed in sudden infant deaths. Adrenal cortex hypofunction was indirectly confirmed by the presence of thymomegaly. Long-lasting glucocorticoid deficiency in secondary hypocorticoidism may cause sudden death following minor environmental causes 13. In the present study hypoplasia of the adrenal glands was found on 11 of 56 patients with thymic hypertrophy. However, one patient with thymic hypertrophy show nodular hyperplasia of the adrenal cortex, meanwhile adrenal tumors (adrenocortical adenoma and adrenal pheochromocytoma) were seen in three patients with thymic hypertrophy, which haven’t been documented. The thymus from a 27-year old man who died of coronary artery thrombosis and accompanied by adrenal pheochromocytoma was weighted even to 183g. It seems development and involution of the thymus closely relates with function of the adrenal glands and various hormones. The influence is complicated and maybe varies with age or other body conditions.
SUDAs due to acute hemorrhagic pancreatitis had been reported where diagnosis could not be made until autopsy. Tsokos et al. reported 27 cases of acute pancreatitis that presented as sudden unexpected death. In all cases, the diagnosis was first made at autopsy and the majority of affected individuals died during the very early phage of the disease 14, 15. In the present study, there were 6 patients whose cause of death was attributed to acute hemorrhagic pancreatitis. All of them were males and in the age ranging from 20 to 53 years. Marked male predominance is consistent with Shetty’s study, in which the male to female ratio of the 7 SUDAs due to acute hemorrhagic pancreatitis was 6:1 16. We also found more SUDAs were caused by acute hemorrhagic pancreatitis in patients with thymic hypertrophy than without it. Until now, the relationship between thymic hypertrophy and acute hemorrhagic pancreatitis has not been documented. Although much has been known regarding the risk factors, the exact trigger events or pathogenesis still remains obscure 16. Local inflammation caused by activation of trypsinogen to trypsin, followed activation of inflammatory mediators and released large amounts of oxygen radicals played a key role in pancreatic necro-inflammatory injure 17. The thymus is a specialized primary lymphoid organ of the immune system. The malfunction of the thymus may induce abnormal inflammatory/immune reaction, which maybe increases the risk of acute hemorrhagic pancreatitis in the patients with thymic hypertrophy. Likewise, infectious diseases and immune-related diseases account for a higher proportion of deaths in patients with thymic hypertrophy than those without it.
The thymus is a complex epithelial organ composed of heterogeneous cell types expressing different CKs 18. In this study, no SUDA-related markers were found. There is a discrepancy between our results of the CK expression and those published previously 19, 20, 21. Majority previous studies focused on infant and child thymuses, and few cases of immunohistochemical examinations on adult thymuses were documented. It had been found that there were changes of cytokeratin expression in epithelial cells at various stages of thymus involution, which may be related to the aging decline in proliferation of thymocytes 19. On the other hand, our study indicated that CKs showed distinctly different expression patterns in individuals who had different death causes and different disease background. It seems that morphology and molecular characteristics of the hypertrophic thymuses in adults are closely related with body conditions. Each thymus hypertrophy has its own mechanism which is different among individuals, who have complicated combinations of disorders and/or lesions. Likewise, it is not surprising that Smith didn’t find lymphoid follicles in any of the 100 adult thymuses even with immune-related diseases, when many other authors had found an increase in lymphoid follicles in autoimmune diseases in humans 10. In the present study, among the 5 patients with autoimmune diseases, only one (with Crohn’s disease) presented lymphoid follicles in the hypertrophic thymus. The relationship between thymic lymphoid follicles and immune-related diseases, as well as dynamic biomarkers during thymus involution and various disease conditions, a large-scale systematic studies maybe necessary, special on hypertrophic thymuses in adults.
In summary, adults with thymic hypertrophy have significantly increased risk of SUDA at young age. In our opinion, instead of a disease entity “status thymico-lymphaticus” is a systematic abnormality with thymic hypertrophy as a feature involving mainly immune and/or cardiovascular system, probably caused by gene mutations. Development and involution of the thymus maybe relates with function of the endocrine organs and hormones. The patients with thymic hypertrophy show diversity in histology, disease conditions and causes of death. In most cases enlargement of the thymus could not be diagnosed during life until postmortem. How to effectively screen this asymptomatic but potentially lethal condition remains a problem. On the other hand, the hypertrophic thymus would maybe be used as an indicator of some immune-related diseases and cardiovascular diseases in the clinical diagnosis.
References
- 1.Steward D J. (2013) Sudden unexpected death during pediatric anesthesia: from status thymico-hymphaticus to silent cardiomyopathy. Paediatr Anaesth. 23, 1101-1103.
- 2.Gullach A J, Risgaard B, Lynge T H. (2015) sudden death in young persons with uncontrolled asthma – a nationwide cohort study in Denmark. BMC Pulm Med. 15, 35-42.
- 3.Kendall M D, Johnson H R, Singh J. (1980) weight of the human thymus gland at necropsy. , J Anat; 131, 483-497.
- 5. (1998) National collaborative group on organ weight research program. Weights of various organs in Chinese people. , ChineseJPathol; 17, 111-114.
- 6.Wang J X, Li B X, Chen R S. (1995) Reference values of main internal organs for Chinese. Chinese J Radiol Medicine & protection. 15, 248-254.
- 7.Zhu R, Huang H, Zhang H. (2006) Prognostic analysis in chronic hepatitis B patients: a retrospective study of 216 cases about Scheuer scores, in situ expression of viral antigens and tissue hepatitis B virus DNA levels. Liver Int. 26, 82-89.
- 9.Sutherland J S, Goldberg G L, Hammett M V. (2005) Activation of thymic regeneration in mice and humans following androgen blockade. , J Immunol;175: 2741-2753.
- 10.Smith S M, Ossa-Gomez L J. (1981) A quantitative histologic comparison of the thymus in 100 healthy and diseased adults. , Am J Clin Pathol;76: 657-665.
- 11.Nishio H, Iwata M, Suzuki K. (2006) Postmortem molecular screening for cardiac ryanodine receptor type 2 mutations in sudden unexplained death: R420W mutated case with characteristics of status thymico-lymphatics. , Circ J; 70, 1402-1406.
- 12.Corrêa R R, Espíndula A P, Silva R C. (2012) Morphologic analysis of fetal stress organs in different causes of perinatal death. Fetal Pediatr Pathol;31:. 30-38.
- 13.Tsibel B N, Bochkareva A K. (1998) Functional morphology of adenohypophysis thymus and adrenal cortex in sudden death infant death syndrome. , Arkh Patol; 60, 23-27.
- 14.Tsokos M, Braun C. (2007) Acute pancreatitis presenting as sudden, unexpected death: an autopsy-based study of 27 cases. Am J Forensic Med Pathol. 28, 267-270.
- 15.M Di Paolo, Marradi I. (2006) Haemorrhagic complication of acute necrotizing pancreatitis presenting with sudden death. J Clin Forensic Med. 13, 271-273.
- 16.Shetty B S, Boloor A, Menezes R G. (2010) Postmortem diagnosis of acute haemorrhagic pancreatitis. , J Forensic Leg Med;17: 316-20.
- 17.Tadao M, Yuji O. (2004) Role of free radicals in the development of severe acute pancreatitis. Nihon Rinsho. 62, 2015-2020.
- 18.Tt Kuo. (2000) Cytokeratin profiles of the thymus and thymomas: histogenetic correlations and proposal for a histological classification of thymomas. Histopathology. 36, 403-414.
- 19.Masunaga A, Sugawara I, Nakamura H. (1997) Cytokeratin expression in normal human thymus at different ages. , Pathol Int; 47, 842-847.
Cited by (1)
- 1.Mussabekova Saule Amangeldievna, Burkova Elena Igorevna, Dobler Kristina Ergardovna, Muldasheva Balzhan Smailovna, Atmtaev Zhan Zhumagulovich, 2024, Sudden infant death syndrome as a result of thymic-lymphatic dysgenesis, Journal of Clinical Medicine of Kazakhstan, 21(1), 97, 10.23950/jcmk/14266
