Call to Action: The Need for Adverse Drug Event (ADE) Standardization and Codification Through Improved ADE Definitions, Documentation and Mapping, as well as More Refined Medication Definitions
Abstract
Information on adverse drug event (ADE) assessment and prevention within Electronic Health Records (EHRs) is difficult for clinicians to use and produces wide-ranging results. Challenges include inconsistent ADE and drug product definition and documentation, workflows, terminology standardization, interoperability, and clinical decision support (CDS) to inform clinical decision-making within EHRs. These factors contribute to care issues for clinicians, such as alert fatigue and provider burden for clinicians and medical errors, patient harm, and even death for patients. Clinicians play the primary role in documenting, reviewing, detecting, and preventing ADEs within EHRs. It is essential that clinicians, clinical informaticists, nursing informaticists, pharmacy informaticists, and the health informatics profession understand the current electronic ADE paradigm to advocate for improved detection and prevention of ADEs within EHRs.
Author Contributions
Academic Editor: Dumrul Gulen, Assoc. Prof. PHD Head of Tumor Biology & Immunology department
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2025 John McCue, et al
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Information on adverse drug event (ADE) assessment and prevention within Electronic Health Records (EHRs) is difficult for clinicians to use and produces wide-ranging results. Challenges include inconsistent ADE and drug product definition and documentation, workflows, terminology standardization, interoperability, and clinical decision support (CDS) to inform clinical decision-making within EHRs. These factors contribute to care issues for clinicians, such as alert fatigue and provider burden for clinicians and medical errors, patient harm, and even death for patients. Clinicians play the primary role in documenting, reviewing, detecting, and preventing ADEs within EHRs. It is essential that clinicians, clinical informaticists, nursing informaticists, pharmacy informaticists, and the health informatics profession understand the current electronic ADE paradigm to advocate for improved detection and prevention of ADEs within EHRs.
ADEs are defined as preventable harm experienced by a patient due to exposure to medication.1 ADE’s account for more than 1.5 million emergency department visits and nearly 500,000 hospitalizations in the United States annually.2 The economic impact of ADE treatment is substantial as well. While there is no consensus on how to analyze costs associated with ADEs, estimates from 2013 state costs in the United States may cost up to 30.1 dollars annually, with costs likely to be even more currently.3In hospital settings, drug allergies are among the most serious types of ADEs. The term “allergy” is used by clinicians, patients and patient historians to denote both immunologically and non-immunologically ADEs to particular agents that they believe will reoccur on repeated exposure. The term “allergy” is commonly misused to refer to any ADE. This misnomer is integrated into almost all (both paper and electronic) patient intake processes which contributes significantly to both clinical and patient misunderstanding, as well as analytical confusion and obfuscation.
ADE CDS alerts play a vital role in preventing patient harm. However, using ADE information to prevent an adverse response or for choosing appropriate evidence-based treatment is often compromised by a lack of quality data, incomplete data, and reliance on unstructured data. Without quality data and usable recommendations, ADE CDS alerts are often too broad and less capable, resulting in greater exposure to more costly, less effective, or even inappropriate treatment, thereby causing patient harm or even death.
ADE CDS can also result in excessive alerts requiring and prolonging clinical assessments. One study reviewed 26,000 drug-drug interaction alerts and found a median time for review about 8 seconds/alert.4
ADE Data Capture within Clinical Workflows
To capture ADE information, clinical ADE reporting within an EHR exists in the ADE module and may often only include a suspected causative agent without adequate documentation of a specific reaction or its severity. For complete information to be captured, ADE health data must be standardized using understandable and transferrable (i.e., interoperable) data standards and terminology between systems. Current practices leverage varying terminologies, thus obscuring results. The wide variation in common terminologies within ADE documentation are detailed in Table 1. “Lack of standardization between systems likely undermines the comparability of the ADE data being generated, and limits meaningful data aggregation across cohorts.”5 Industry efforts have made recent strides in improving interoperability of health data using USCDI, but complete ADE information is still not captured within EHRs, preventing proper capture and transfer between systems Figure 1.
Figure 1.Categorization of Drug Product effects in both active and inactive ingredients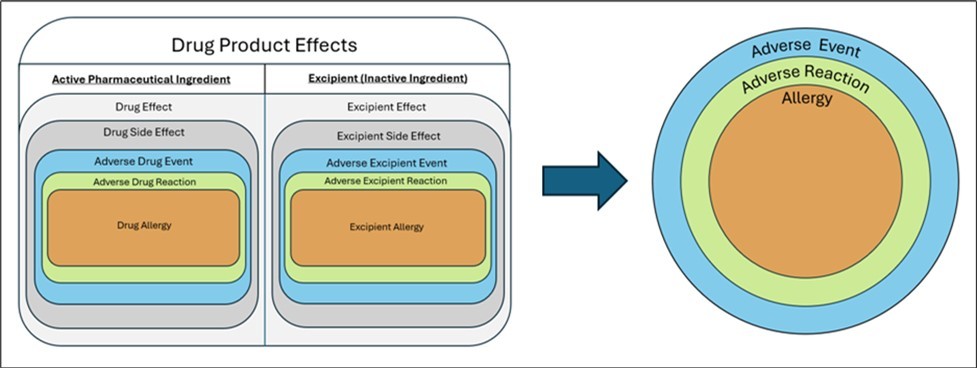
| Terminology | Description | Relevance to ADE Capture |
|---|---|---|
| National Drug Code (NDC)6 | A universal numerical product identifier for drugs | Standardizes drug product information (product name, NDC number, active ingredients, strength, routes of admission, major drug class, FDA approved application number) |
| Systematized Nomenclature of Medicine – Clinical Terms (SNOMED-CT)7 | Medical terminology standard required for clinical health information exchange | Standardizes medical information (allergy, reactivity, severity, medical care concepts) |
| RxNorm Normalized Codes and Names (RxNorm)8 | Terminology standards for clinical drugs | Standardizes generic and brand drug names; supports interoperability between drug terminologies and pharmacy knowledge base systems |
| Longitudinal Observation Identifiers Names and Codes (LOINC)9 | Terminology standards for laboratory test orders and results | Standardizes laboratory data, such as allergy and immunologic testing data |
| International Classification of Diseases (ICD)10 | Global standards for classifying diseases, injuries, and health conditions | Facilitates reporting of drug-related information and standardizes ADE information. Also used in billing and quality data capture. |
| Fast Health Interoperability Resources (FHIR)11 | Standard for how healthcare information can be exchanged regardless of storage in initial location; developed by Health Level 7 (HL7) as an evolution of HL7 healthcare standards and Clinical Document Architecture (CDA) | Standards for data capture and data information exchange related to ADEs |
| United States Core Data for Interoperability (USCDI)12 | Health data classes and constituent data element for nationwide, interoperable health information exchange | Provides standards for data information exchange related to allergies within the United States |
Another common issue with ADE documentation is the use of free-text documentation, producing unstructured data not associated with any data terminology. Free-texting documentation refers to manual input of texting documentation into an EHR instead of choosing from standardized data, search engines, or algorithms embedded within ADE workflows. “Approximately 29 percent of reactions are entered as free text, and a third of allergy entries lack reaction descriptions entirely.”13 Clinicians often resort to free-texting ADE information due to a lack of appropriate structured information, time constraints, or workflow inconsistencies. By free-texting, information related to ADEs cannot be reliably incorporated into CDS or other cross-cutting areas within an EHR.
Once ADE information is captured within an EHR platform, it is then analyzed in medication processes, such as ordering, dispensing, reconciliation, and reviews. This analysis is commonly completed by CDS, which is informed by EHR data, rule engines, and drug product databases. However, ADE analysis is highly customized across health organizations and not standardized. Figure 2 details the current state for CDS ADE review.
Figure 2.Current State for CDS ADE Review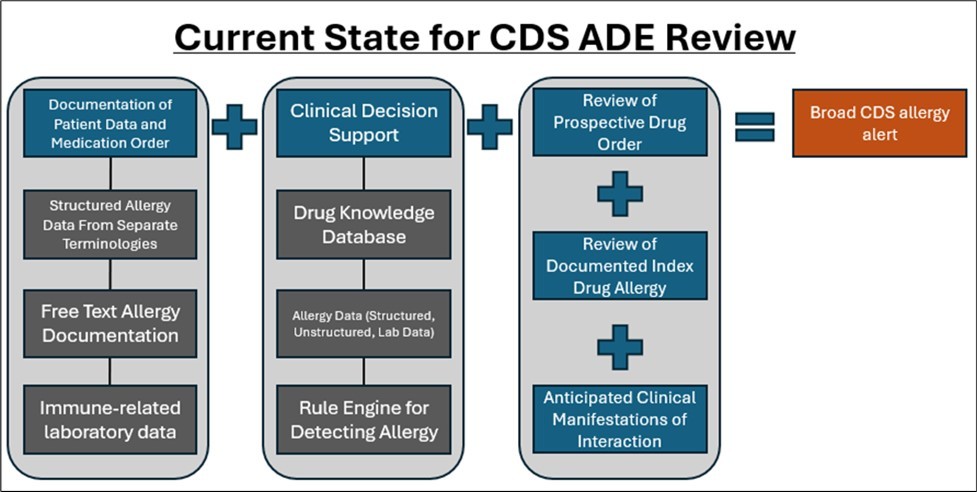
Datasets in commercial drug product databases play a large role in ADE detection and prevention and are supplemental to EHR data. These datasets contain information related to formulations, interactions, ADE responses, severity, reactivity, etc. Common datasets include First Databank, Multum, Micromedex, MediSpan, and Gold Standard. 14, 15, 16, 17, 18 When a healthcare entity integrates a clinical information system or EHR into their organization, they either choose their preferred dataset or are provided with the dataset used by their CDS or EHR vendor. These datasets may also have prepopulated ADE CDS options for configuration within their system, such as drug classes, types of alerts, and/or severity of alerts. While these datasets have played a significant role in improving ADE detection and prevention, their intersystem content variability – and their lack of validated clinical assessments - contribute to a lack of standardization across ADE CDS drug interaction.19
Clinical Informaticists Role in Ade Data Standardization
Clinical informaticists bridge many professions within healthcare through the enhancement of information technology and interprofessional collaboration. ADE detection and prevention requires a holistic approach to better apply knowledge and expertise from across the healthcare spectrum and optimize information technology processes.
While clinicians play the primary role in collecting information and executing decisions related to ADE detection and treatment, clinical informaticists play key roles in developing, maintaining, and updating EHRs CDSs. Some roles and responsibilities of clinical informaticists include bridging the technical and clinical world, mapping data across different terminologies, monitoring and maintaining datasets, analyzing data to identify trends, and developing tools, such as CDS tools, to detect and prevent ADEs. Clinical informaticists play a profound role in ADE prevention through EHR maintenance and upgrades; implementing tools to integrate data into EHRs; training a clinical workforce on information technology aspects of workflows; implementing and optimizing CDSs; performing root-cause analyses related to ADEs; and participating in medication safety committees. Clinical informaticists also enhance collaboration among healthcare professionals for creating solutions integrating existing workflows or reducing redundancies across ADE-related patient care.
Barriers and challenges to optimizing ADE detection and prevention are evident throughout healthcare, especially within EHR functionalities. While CDS is beneficial, it can become burdensome, contributing to provider burden and alert fatigue when not optimized for clinical workflows. Estimates show up to 88% of CDS alerts are overridden or bypassed for being too broad or not tailored towards patient-relevant information.20 Commercial drug product datasets vary in data and data structure, preventing a universal approach to utilization within CDS processes. Current CDS processes also contain limited drug product formulation information in their ADE reviews, potentially creating misleading conclusions. To complicate both of these issues further, terminology and education related to practices is inconsistent. Targeting education to standardize ADE definitions and prevention methods is a crucial effort to improve ADE reporting, prevention, and detection.21
Value sets are a common tool maintained and executed by data analysts and clinical informaticists. Value sets provide groupings of codes relating to a clinical concept and are often designed by regulatory bodies, health IT vendors, or in-house by healthcare organizations.22 They are used within EHRs and other clinical tools for such use cases as population health management, clinical trials, quality reporting, and CDS. They must be developed, updated, and maintained as experience and knowledge progresses. Some of the more common value sets from regulatory agencies include CDS eClinical Quality Measures and the National Cancer Institute.22
Solution
For ADE information to produce more relevant and accurate alerts for CDS, clinicians must have a proper infrastructure to produce structured data with accurate, applicable insights. Studies indicate CDS must be user-friendly, compliant with clinical guidelines, integrable into existing workflows, properly embedded with supporting literature, and provide accurate real-time alerts in the prescription process to prevent practitioner burden. To identify a solution for ADE data quality, United States Pharmacopeia (USP) convened a panel of experts to investigate the problem and propose solutions. Through clinician interviews, expert discussion, extensive literature review, and system analyses, the panel identified potential solutions for standardization and interoperability of ADE information.
To produce an ADE solution, the expert panel identified the importance of establishing a common set of standards for ADE assessment and documentation. This provides an interoperable dataset that follows a patient to other EHR systems throughout the patient’s life. Documentation of ADEs should take place in a structured manner with maximum granularity. Where possible, all specific medication information (including product formulations and dosing) should be captured, along with specific clinical manifestations of the reaction, including timing and severity. The existence of co-occurring clinical and environmental conditions, as well as data elements regarding times of exposure and times of appearance of clinical manifestations should be recorded when possible.
One solution to enhance terminology standardization for ADEs would be the development of an ADE value set and establishment of a mechanism to evaluate, add to, and update the value set as necessary. An ADE value set should be structured to allow the specific chemical (when identified), or at least the drug product formulation causing the clinical manifestation, to be cross-referenced with the prospective drug order and result in a precise CDS message to the clinician on the potential risk of a reaction re-occurring, as well as associated literature to support. An ADE value set should also incorporate concepts from varying terminologies to ensure that complete information from ADEs is included, such as active ingredients, excipients, clinical manifestations, severity, reaction, reactivity, and other relevant content, and appropriately captured. An illustration of the ADE value set within a CDS workflow is detailed in Figure 3.
Figure 3.Value Set Embedded in CDS ADE Review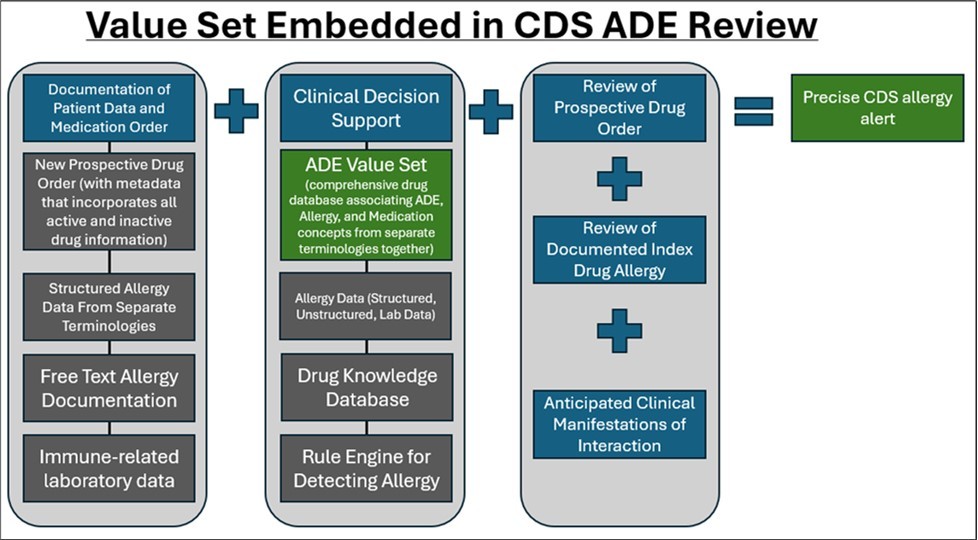
In creating a value set, the goal is to strengthen ADE detection by electronically mapping well -documented drug products to potential clinical manifestations, with an outcome of improving patient safety. By electronically mapping drug products to potential clinical manifestations, clinicians will also have more accurate information to establish whether a clinical manifestation is likely to occur due to a specific chemical entity (active ingredient vs excipient vs the combination). The value set also features standardized, interoperable codes to improve clinical decision making, interoperability of patient ADE information across EHR’s, efficacy and quality of alerts, and reduction of excessive drug alerts.
The ADE value set is designed to address four specific aims to:
utilize standard terminology, allowing an exchange of coded sets across multiple platforms and settings (interoperability) to ensure ADE information is accurate, accessible, and visible to all healthcare practitioners for treatment decision making.
Utilize a value set to expand ADE documentation to include both accurate, complete drug product information and the related clinical manifestation in standardized language attached to terminology.
Add a layer of medication safety through a more targeted clinical decision support message for the clinician on possible future ADE’s based on a patient’s current medical history.
Improve data collection and information that accurately depicts the frequency of ADE’s and reveals previously undocumented ADE’s.
While an ADE value set brings forward much potential, there are known challenges in creating and maintaining a value set. Value set creation is time consuming and requires a manual process to identify, create, and maintain mapping of data elements by informaticists and medical data analysts. For the value set to be effective, data must be appropriately mapped from different data standards and formats, although challenges related to mapping different terminologies to each other are known. The creation of ADE value sets also requires considerable stakeholder engagement throughout the health informatics ecosystem, including terminologists, EHR vendors, informaticists, clinicians, and other key stakeholders. Ownership of an ADE value set could exist from a private entity, healthcare organization, standard setting organization, or regulatory agency, which would ensure maintenance and optimization of the value set. Furthermore, this value set must be constantly reviewed and updated for these reasons as well as the emergence or discovery of new ADE’s.
An ADE value set also has the potential to be scaled for additional use cases and functionalities. Estimates show that nearly 69% of ADEs can potentially be prevented by tailoring ADE CDS with granular information incorporating more patient-specific information.23
Increased adoption of new technologies, such as machine learning (ML) and artificial intelligence (AI), should also provide further insights into ADE prevention and detection 24, 25, 26.
Conclusion
USP aims to improve clinical workflows and patient safety through the development, refinement, and use of an ADE Value Set– including all subsets: Adverse Drug Reactions, Drug Allergies, Drug Side Effects, and other Drug Intolerances. This value set tool will be incorporated into EHR CDS and improve identification, documentation, and use of patient ADE information by providers, thereby decreasing provider burden. Such a resource should reduce the risk of repeat reactions in patients who have a history of an ADE, as well as prevent the onset of a new ADE for a patient.
USP calls on clinical informatics stakeholders and other stakeholders across the healthcare industry to collaborate on a solution that prevents ADE’s and improves the usability of allergy information within EHR CDS. To do this, EHR systems must create an interoperable system that analyzes all pertinent drug product information (both active pharmaceutical ingredient information and excipient information) used by practitioners and IT systems. If EHR’s incorporate an ADE value set into their systems, clinicians may be presented with fewer and more accurate ADE alerts. This value set should be available industry-wide with assistance from regulatory bodies, knowledge vendors, and EHR vendors.
References
- 1.S U. (2014) Department of Health and Human Services, Office of Disease Prevention and Health Promotion. Author. Retrieved from https://odphp.health.gov/sites/default/files/2019-09/ADE-Action-Plan-508c.pdf , Washington, DC:
- 2.Centers for (2024) Disease Control and Prevention. FastStats: Medication safety data. Accessed , CDC. Published April 17.
- 3.Sultana J, Cutroneo P, Trifirò G. (2013) Clinical and economic burden of adverse drug reactions. Retrieved from https://pmc.ncbi.nlm.nih.gov/articles/PMC3853675/ , Journal of Pharmacology & Pharmacotherapeutics 4, 73-77.
- 4.Page N, Baysari M T, Westbrook J I. (2017) A systematic review of the effectiveness of interruptive medication prescribing alerts in hospital CPOE systems to change prescriber behavior and improve patient safety. , Int J Med Inform 105, 22-30.
- 5.Page RL 2nd, O'Bryant C L, Cheng D. (2016) Drugs That Prolong the QT Interval and/or Induce Torsades de Pointes: A Comprehensive Review. Crit Pathw Cardiol. 15(4), 183-202.
- 6.U S Food, Administration Drug. (2025) National Drug Code database background information. https://www.fda.gov/drugs/development-approval-process-drugs/national-drug-code-database-background-information , FDA. Accessed February 26.
- 8.National Library of Medicine (2025) . https://www.nlm.nih.gov/research/umls/rxnorm/index.html , RxNorm. NLM. Accessed February 26.
- 11. (2025) Office of the National Coordinator for Health Information Technology. What is FHIR?. ONC. Published 2019. Accessed 2019-08.
- 12. (2025) Office of the National Coordinator for Health Information Technology. United States Core Data for Interoperability (USCDI). https://www.healthit.gov/isp/united-states-core-data-interoperability-uscdi , ONC. Accessed
- 13.Zhou L. (2022) Improving allergy documentation and clinical decision support in the electronic health record - Final report. Agency for Healthcare Research and Quality. Published. Accessed
- 15.Pharmacy. (2025) Oracle Health. Accessed. https://www.oracle.com/health/service-lines-departments/pharmacy/
- 18.Elsevier. (2025) Gold Standard Drug Database. Accessed. https://elsevier.health/en-US/marketing/drug-information/gold-standard-drug-database-ppc
- 19.Fung K W, Kapusnik-Uner J, Cunningham J, Higby-Baker S, Bodenreider O. (2017) Comparison of three commercial knowledge bases for detection of drug-drug interactions in clinical decision support. , J Am Med Inform Assoc 24(4), 806-812.
- 20.Sijpe G Van De, Quintens C, Walgraeve K. (2022) Overall performance of a drug–drug interaction clinical decision support system: Quantitative evaluation and end-user survey. , BMC Med Inform Decis Mak.22: Article 48, 10-1186.
- 21.National Library of Medicine. Value Set Authority Center (VSAC) (2025) . https://vsac.nlm.nih.gov/
- 22.Shalviri G, Mohebbi N, Mirbaha F. (2024) Improving adverse drug event reporting by healthcare professionals. Cochrane Database Syst Rev. 10(10), 10-1002.
- 23.Shah S N, Seger D L, Fiskio J M, Horn J R, Bates D W. (2021) Comparison of medication alerts from two commercial applications in the USA. , Drug 44(6), 661-668.
- 24.Bates D W, Levine D, Syrowatka A. (2021) The potential of artificial intelligence to improve patient safety: a scoping review. , NPJ Digit Med 4, 54-10.
